To the Editor:
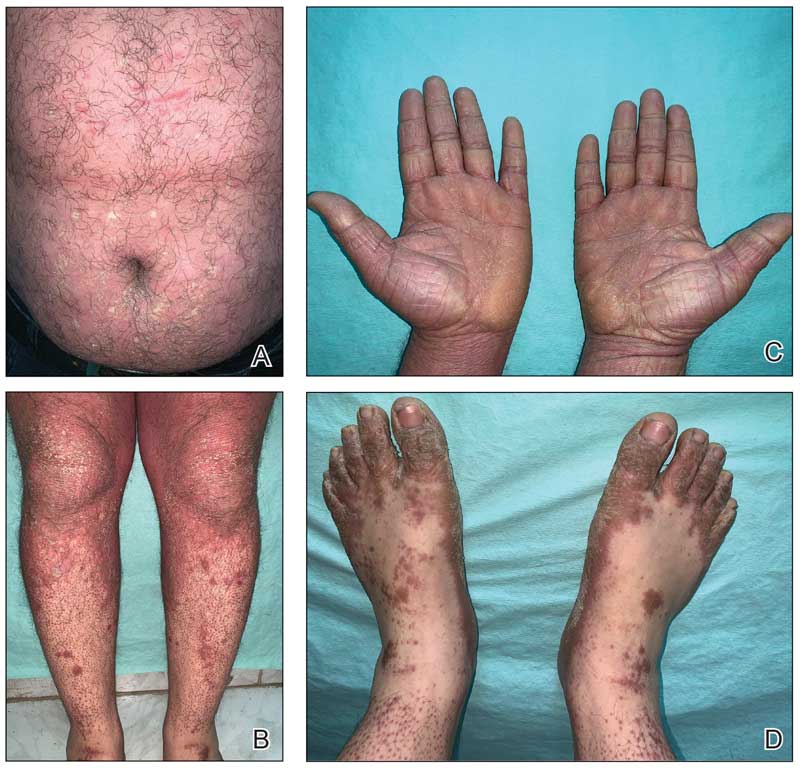
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed orange-red erythroderma (Figure 1A) with islands of sparing, keratotic follicular orange-red papules on both legs and feet (Figure 1B), well-defined waxy palmoplantar keratoderma (Figures 1C and 1D), and fine scales on the face and scalp. The clinical and laboratory workup were normal, including a negative test for HIV infection.
Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed irregular epidermal hyperplasia with thick suprapapillary plates and hypergranulosis (Figure 2A) along with alternating orthokeratosis and parakeratosis in vertical and horizontal directions (checkerboard parakeratosis)(Figure 2B). Follicular plugging with shoulder parakeratosis also was seen. The dermis showed a mild, superficial, perivascular lymphohistiocytic infiltrate. These features were diagnostic of pityriasis rubra pilaris (PRP). The patient received acitretin 25 mg/d and methotrexate 17.5 mg/wk (0.4 mg/kg/wk) and showed marked improvement after 2 months of therapy.
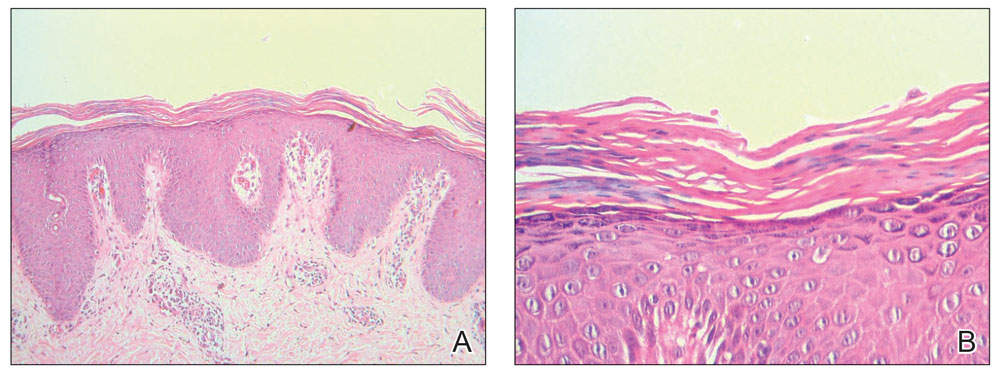
FIGURE 2. A, Irregular epidermal hyperplasia with thick suprapapillary plates, hypergranulosis, and alternating orthokeratosis and parakeratosis in vertical and horizontal directions. The underlying dermis showed a mild, superficial, perivascular lymphohistiocytic infiltrate (H&E, original magnification ×100). B, Characteristic checkerboard parakeratosis was shown at higher magnification (H&E, original magnification ×400).
Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after the Sinopharm BBIBP-CorV vaccine including psoriasis, lichen planus, and pityriasis rosea. Skin manifestations occurred sporadically, as some happened after the first or second dose or even after booster doses. The exact pathogenic mechanism(s) underlying the development of these conditions following vaccination still are not understood, though they may be attributed to COVID-19 vaccine–induced immune dysregulation.6
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.