The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Staff Physician
Cleveland Clinic
Cleveland, OH
Dr. Balakrishnan reported no relevant financial disclosures.

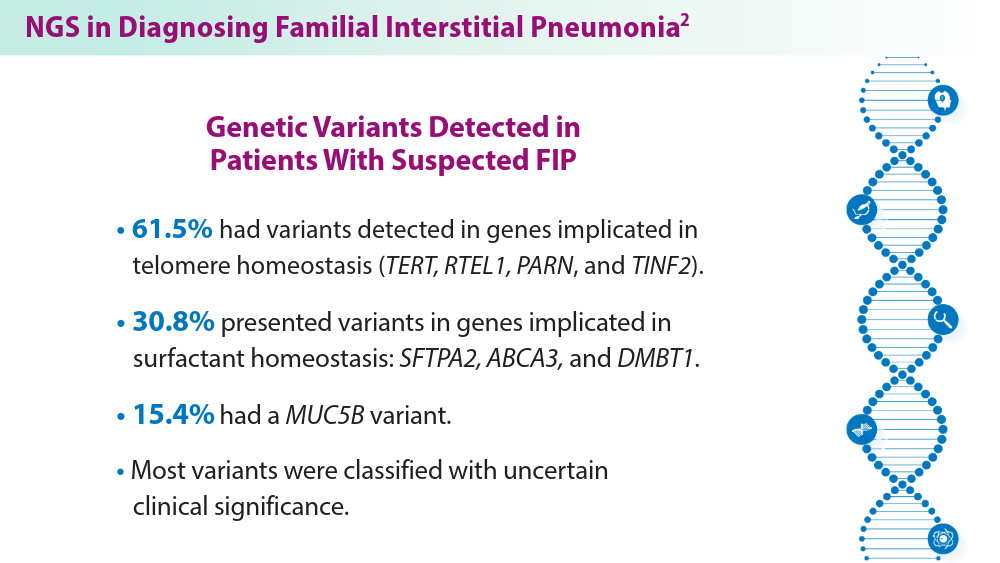
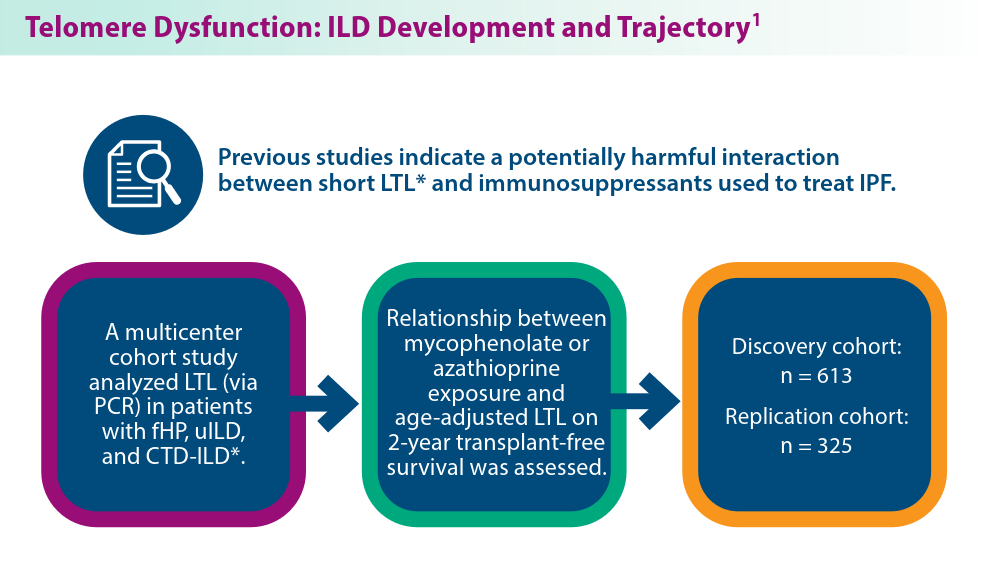
ILDs often require pharmacological therapy to prevent progressive loss of lung function. The initial treatment choice is determined by the ILD subtype.1 Telomeres shorten with cellular replication and the natural aging process, and telomere dysfunction has been linked with ILD development and disease progression.1 A pharmacogenomic relationship may exist between immunosuppressive treatment and shorter leukocyte telomeres. Historical use of immunosuppression is associated with worse survival for patients with IPF with short age-adjusted telomere length.1 Genetic factors may contribute to ILD development, as seen in familial interstitial pneumonia (FIP).2 More than 10 gene mutations are associated with FIP.2 When FIP is suspected, next-generation sequencing (NGS) helps facilitate a targeted gene panel with a known familial ILD association. Initial studies of familial clustering of ILD led to the discovery of gene mutations implicated in telomere homeostasis (telomere-related genes) and surfactant homeostasis (surfactant-related genes).2 The disease phenotype in families was not limited to IPF, but included various fibrosing diseases, all of which have the potential for progressive pulmonary fibrosis. Genetic and epigenetic (eg, viral, exposure) underpinnings highlight the complexity of ILD etiology, with mutations in telomere-related and surfactant-related genes contributing to pulmonary fibrosis phenotypes.3 As research advances, understanding these genetic, environmental, and molecular mechanisms holds promise for tailored therapeutic strategies for ILD management.
1
-
-
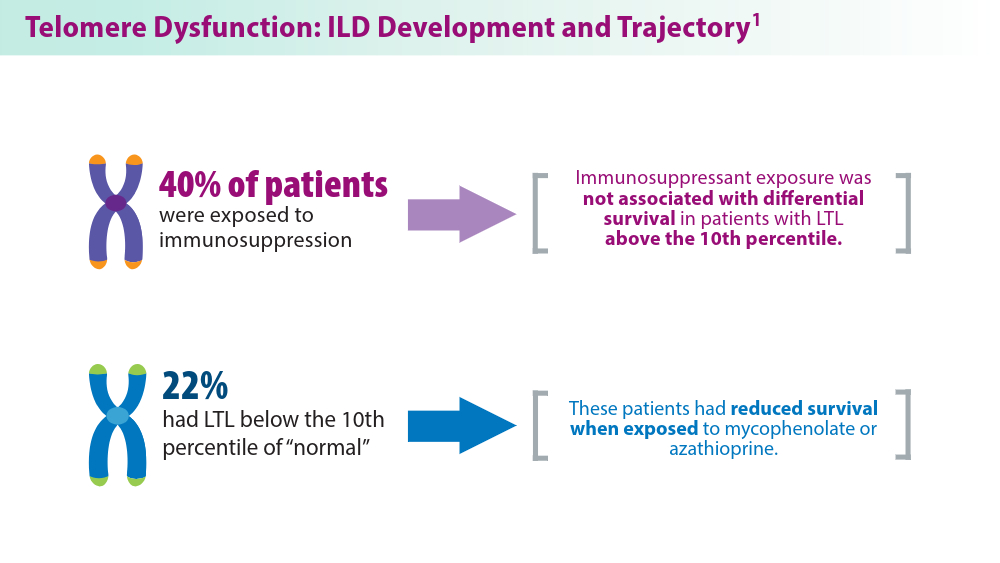
Conclusion: LTL may be a clinically viable genomic marker to aid treatment decisions for patients with fHP and uILD.
-
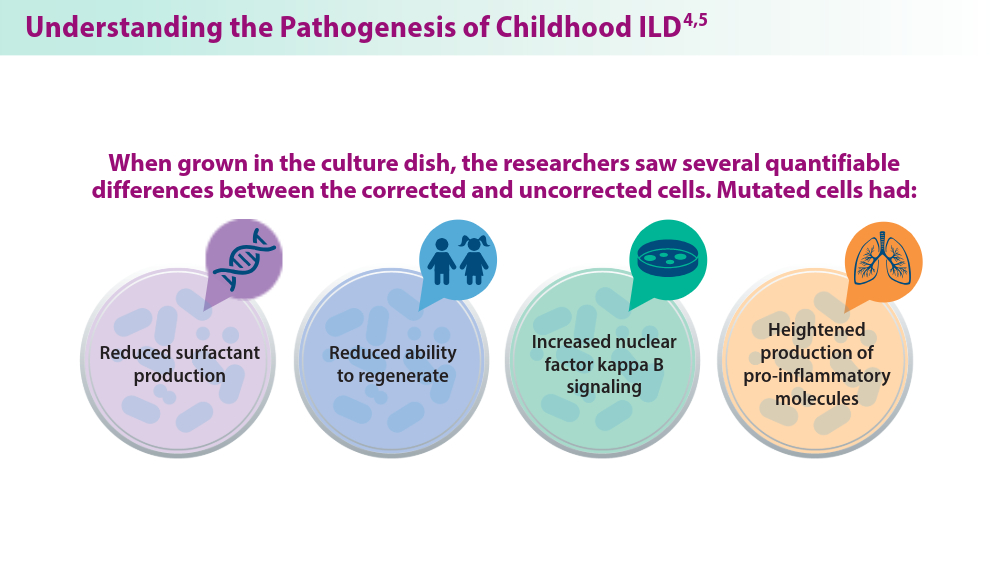
Researchers recently created a model using CRISPR/Cas9 gene editing to study the effects of ABCA3 mutations. AEC2s were generated from the induced pluripotent stem cells (iPSCs) of children carrying ABCA3 mutations. Both corrected and uncorrected cells were engineered for comparison. ABCA3 gene mutations are the leading genetic contributors to childhood interstitial lung disease(chILD). Limited treatment options are available for patients with ABCA3 mutations because the disease pathogenesis is not well understood, partially due to an inability to obtain AEC2 cells from children with ILD. These results allowed the researchers to better understand the mechanisms of chILD, opening the door for targeted therapies in the future.
-
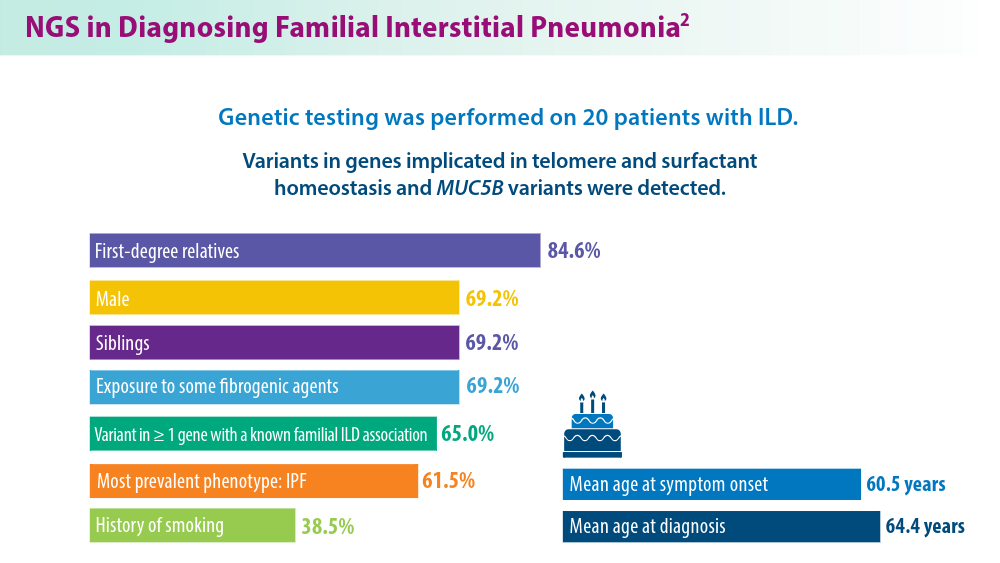
A 10-fold increase in the prevalence of ILD among families of patients diagnosed with IPF suggests a genetic predisposition to ILD. Investigations into familial ILD have uncovered variants in genes associated with the maintenance of telomeres and surfactant homeostasis. These genetic variations, along with several genetic polymorphisms, have been implicated in the disease.
-