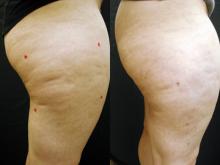
GRAPEVINE, TEX. – An investigational sidelight 3-D optical fiber and 1440-nm Nd:YAG laser produced significant improvement of cellulite with one treatment at 6 months in a study of 15 healthy women.
Cynosure Inc.'s Cellulaze Cellulite Laser Workstation's 1440 wavelength is well absorbed by adipose tissue and water. The side-firing SideLight 3-D optical fiber thermally subcises subcutaneous septa, deplanes fat cells, and heats dermal tissue to promote skin thickening and tightening, said Dr. Bruce E. Katz of the department of dermatology at Mount Sinai School of Medicine, New York.
The 15 women (aged 20-55 years) all had cellulite on their lateral or posterior thighs or buttocks, body mass indexes less than 30 kg/m2, and skin types I-V. Following local anesthesia, two 1.5-mm incisions were made, and the probe was inserted. Subcutaneous temperature was kept at lower than 47° C and surface temperature at lower than 40° C. The Nd:YAG laser delivered 1,000 J per 5- x 5-cm square.
Digital photographic evaluation by two independent observers at 6 months found that 68% of the women had excellent improvement in cellulite; Vectra 3-D imaging demonstrated significant improvement in 65% of them. By physician evaluation, 76% had good or excellent results (69% by patient evaluation). Vectra analysis of skin contour demonstrated an average 47% reduction in depth and 32% reduction in height of skin bulges, Dr. Katz reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Histologically, there was an increase in coarser collagen and elastic fibers in the dermis, noted Dr. Katz, who is also director of the cosmetic surgery and laser clinic at Mount Sinai Medical Center.
Three patients experienced mild ecchymoses, and four had edema lasting less than a week. There were no other adverse events.
"This may be a game changer for the treatment of cellulite," Dr. Katz said.
Cynosure received CE marking certification for Cellulaze in the European Union in February. The U.S. Food and Drug Administration approved an Investigational Device Exemption (IDE) for the device, and a clinical IDE study is currently underway. Regulatory action on a 510(k) submission, filed in late 2010, is currently expected in the first half of 2011, according to a company statement.
Dr. Katz disclosed that he is a Cynosure stockholder.