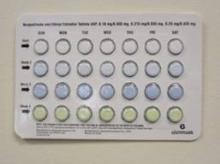
A nationwide recall of seven lots of oral contraceptives containing norgestimate and ethinyl estradiol because of a packaging error that could result in suboptimal protection against pregnancy has been announced by the manufacturer, the Food and Drug Administration announced on Feb. 27.
A statement on the agency’s MedWatch site said that as a result of a packaging error in these lots, "the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy." The FDA advised that women who have been "exposed to" the affected packaging should start using a nonhormonal form of contraception immediately and those who have the product should return it to the pharmacy and contact their physician.
The affected OCs, manufactured by Glenmark Generics, were distributed to wholesalers and retail pharmacies nationwide between Sept. 21, 2011, and Dec. 30, 2011, and contain the following doses of norgestimate and ethinyl estradiol: 0.18 mg/0.035 mg, 0.215 mg/0.035 mg, and 0.25 mg/0.035 mg.
The packaging error that prompted the recall is described as select blisters that are rotated 180 degrees* within the card, which reverses the weekly tablet orientation and makes the lot number and expiration date visible on the outer pouch only, according to the FDA.
A statement issued by Glenmark said the following lot numbers are affected: 04110101, 04110106, and 04110107, with the expiration date of 7/31/2013; 04110114, 04110124, and 04110129, with the expiration date of 8/31/2013; and 04110134, with the expiration date of 9/30/2013.
Adverse events that may be related to the use of these products should be reported to Glenmark Generics, at 888-721-7115 (8 a.m. to 5 p.m. Monday through Friday EST), or to the FDA’s MedWatch Program anytime by faxing 800-332-1078 or visiting its website.
*CORRECTION 3/7/12: The original sentence misidentified the packaging error.