Clinical Review

2014 Update on Contraception
Does immediate postpartum insertion of a contraceptive implant reduce the repeat pregnancy rate in teens? What makes the copper IUD the best...
EXPERT COMMENTARY
Andrew M. Kaunitz, MD
Dr. Kaunitz is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville, and Director of Menopause and Gynecologic Ultrasound Services, UF Women’s Health Specialists–Emerson. Dr. Kaunitz serves on the OBG Management Board of Editors.

It reduces postinsertion pain but not the pain associated with placement, according to this randomized, double-blind, placebo-controlled comparison of ketorolac and placebo. In fact, 22% of patients in the placebo group and 18% in the ketorolac group reported that the pain of injection was as severe as the pain of IUD placement.
Ngo LL, Ward KK, Mody SK. Ketorolac for pain control with intrauterine device placement: a randomized controlled trial. Obstet Gynecol. 2015;126(1):29–36.
Although the use of intrauterine devices (IUDs) is increasing, these highly effective contraceptives remain underutilized in the United States, compared with other developed countries. Concerns about pain with insertion represent one barrier to use.
In a double-blind trial, Ngo and colleagues randomly assigned women presenting for first-time IUD placement from 2012 to 2014 to either:
The injection was given 30 minutes prior to IUD placement.
Pain associated with injection of the study drug, speculum and tenaculum placement, uterine sounding, IUD placement, and postinsertion pain were measured using a visual analog scale from 0 cm (no pain) to 10 cm (worst pain possible).
Of 67 participants (mean age, approximately 27 years; white race, 33%; African-American race, 33%; median parity, 1), pain was similar between ketorolac and placebo arms for all parameters except postinsertion pain, which was 0 cm and 1.3 cm for ketorolac and placebo, respectively, 15 minutes after placement (P<.001).
Although approximately 75% of participants reported that pain from the injection was “not as bad” as the pain from IUD placement, about 1 in 5 indicated that injection pain was equivalent to pain from IUD placement. Regardless of study group allocation, more than 90% of participants reported being satisfied or very satisfied with IUD placement overall, and more than 75% said they would recommend IUD placement to a friend.
More than 90% of women were satisfied with IUD placement, regardless of study allocation
As Ngo and colleagues observe, the analgesic effect of ketorolac peaks 1 to 2 hours after injection. This observation may explain why pain reduction was noted only after IUD insertion. Although ketorolac is not expensive, logistic considerations may make its routine use prior to IUD placement unrealistic in many ambulatory settings. Further, the great majority of participants (>90%) reported being satisfied with their IUD placement experience overall, regardless of study allocation.
Earlier studies suggesting that preplacement oral NSAIDs are ineffective in reducing placement pain involved the administration of analgesia in the clinic less than 1 hour before IUD insertion. I agree with Ngo and colleagues that future trials of oral NSAIDs should focus on administration of the medication prior to arrival at the clinic.
What this evidence means for practice
Findings from this randomized controlled trial provide only limited support for injection of an NSAID prior to IUD placement.
--Andrew M. Kaunitz, MD

Does immediate postpartum insertion of a contraceptive implant reduce the repeat pregnancy rate in teens? What makes the copper IUD the best...
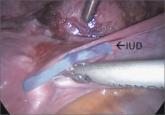
Techniques for managing intrauterine devices that have become embedded, translocated, or perforated
It is slightly more effective, but both devices have a very low contraceptive failure rate, according to this multinational, prospective,...
