News

FDA panel considers contraindications, removal guidelines for Essure
The FDA advisory panel did not make any formal recommendations on Essure, but some called for the creation of a prospective patient registry.

FROM BMJ
The risk of reoperation is more than 10 times greater after hysteroscopic sterilization with the Essure device than after laparoscopic bilateral tubal ligation, according to an observational cohort study published online Oct. 13 in the BMJ.
The finding raises “a serious safety concern” about Essure, a sterilization coil approved in 2002 for hysteroscopic placement into the fallopian tube, and the subject of a recent Food and Drug Administration safety hearing following more than 5,000 adverse event reports.
“While reoperation following sterilization procedure can be related to unintended pregnancy, the similar risk of unintended pregnancy for both procedures in our study indicated that additional surgeries were performed to alleviate complications such as device migration or incompatibility after surgery,” wrote Dr. Art Sedrakyan and his colleagues from Cornell University, New York (BMJ 2015;351:h5162. doi: 10.1136/bmj.h5162).
The Cornell study is believed to be the first to pit Essure against laparoscopic tubal ligation. The investigators compared outcomes of 8,048 patients who underwent hysteroscopic sterilization using the Essure device with 44,278 laparoscopic tubal ligation patients between 2005 and 2013, using a New York state database that captures hospital discharges, outpatient services, ambulatory surgeries, and emergency department records statewide.
Overall, 2.4% of Essure patients, but 0.2% of tubal ligation patients, required reoperation within a year, yielding an odds ratio for Essure of 10.16 (95% C.I., 7.47-13.81), which translates to about 21 additional reoperations per 1,000 Essure patients. Essure patients were eight times more likely to undergo reoperation within 2 years of placement, and six times more likely within 3 years.
Meanwhile, the rate of unintended pregnancy was not statistically different in the two groups, at 1.2% for Essure and 1.1% for tubal ligation within the first year. Essure was also associated with a lower risk of iatrogenic complications within 30 days after surgery, compared with laparoscopic tubal ligation (odds ratio, 0.35).
The use of Essure skyrocketed during the study, from 0.6% of sterilization procedures in 2005 to 25.9% in 2013, with a corresponding drop in tubal ligations. Essure was more likely to be used in women over 40 years old, Medicaid patients, and women with histories of pelvic inflammatory disease, abdominal surgery, and cesarean section. The analysis adjusted for such differences.
Median charges were higher for Essure than for tubal ligation – $7,832 versus $5,068 – despite shorter procedure times, fewer immediate postoperative complications, and less frequent use of general anesthesia.
Although general anesthesia was used less often with Essure, it was still used in about half of patients. “This finding is remarkable in light of the marketing and proposed benefits of avoiding general anesthesia associated with the Essure device,” the investigators wrote.
Tara DiFlumeri, a spokeswoman for Bayer, which manufacturers Essure, said the Cornell study supports the high efficacy rate of Essure. But she also noted that “detection bias” in the study could account for the high reoperation rate identified.
“A required Essure confirmation test is administered 3 months after the procedure to determine whether or not a woman’s fallopian tubes are blocked and she can rely on Essure for birth control. This follow-up test may detect unsatisfactory device placement, resulting in the need for ‘reoperation’ to remove the device and/or complete a tubal ligation if sterilization is still desired. Because there is no confirmation test that could identify potential failure of a laparoscopic tubal ligation procedure, it stands to reason that the comparative reoperation rate would be lower,” she said.
The investigators reported having no financial disclosures. The work was funded in part by the National Institutes of Health and the Food and Drug Administration.

The FDA advisory panel did not make any formal recommendations on Essure, but some called for the creation of a prospective patient registry.
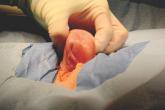
Gynecologic surgeons say hysterectomy isn’t the only option for women who want to remove the Essure device and that in about a third of cases they...
