News
Priorities for determining the etiology of incontinence
Letters from readers
Marjorie L. Pilkinton, MD; Dara Shalom, MD; and Harvey A. Winkler, MD



Dr. Winkler reports that he is a consultant to Astora Women’s Health, Boston Scientific, and Kimberly-Clark. Drs. Pilkinton and Shalom report no financial relationships relevant to this article.

A new FDA approved, over-the-counter option for stress urinary incontinence and several office-based and surgical treatment systems for urinary and fecal incontinence are now available or on the horizon. Here, the essentials.
In this Article
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
Letters from readers
These surgeons review indications and demonstrate patient positioning and their minimally invasive fascia lata harvest technique.

Yes: Median episiotomy was associated with an increased rate of 3rd- and 4th-degree perineal laceration in both nulliparous and multiparous women...
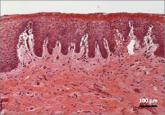
With more than 1 billion menopausal women likely to be affected by vulvovaginal atrophy worldwide by 2025, the need for effective remedies is...
No, provided the patient undergoes careful office evaluation instead, according to this systematic review and meta-analysis Rachaneni S, Latthe P...
