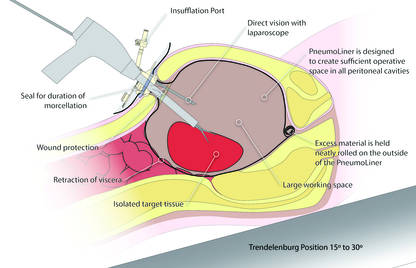
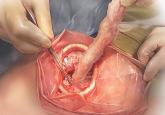
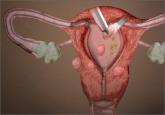
In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| PneumoLiner tissue containment device | | PneumoLiner device placement |
| | |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
As surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.