Developing quality measures
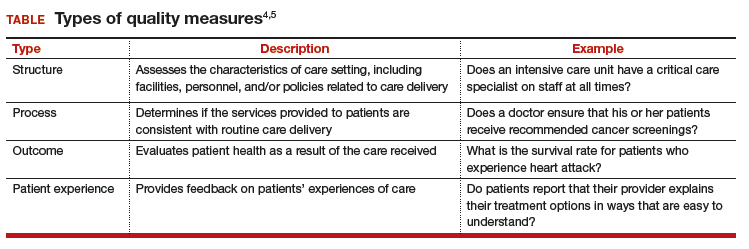
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.
Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1