In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.
What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
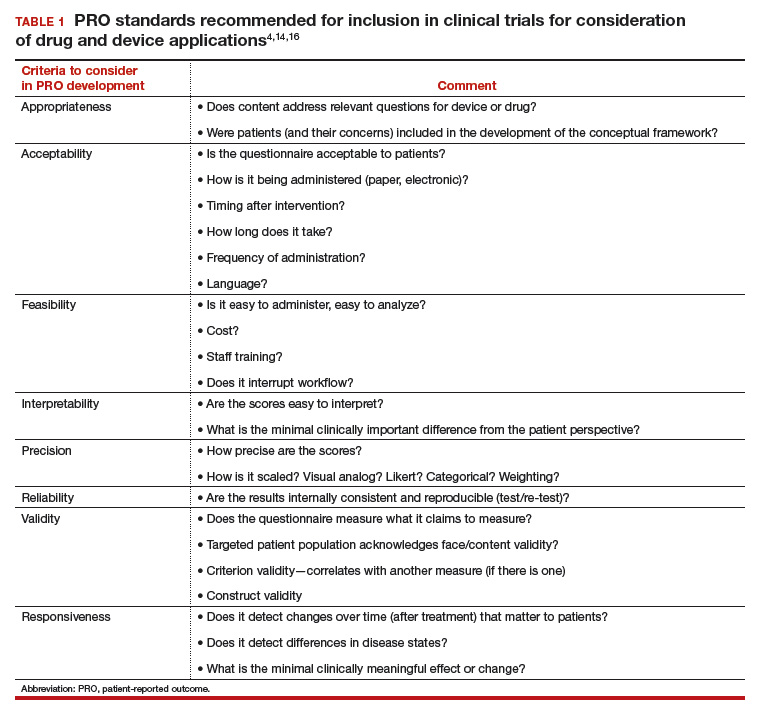
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15