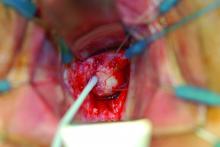
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.