Our approach to pharmacologic cervical ripening
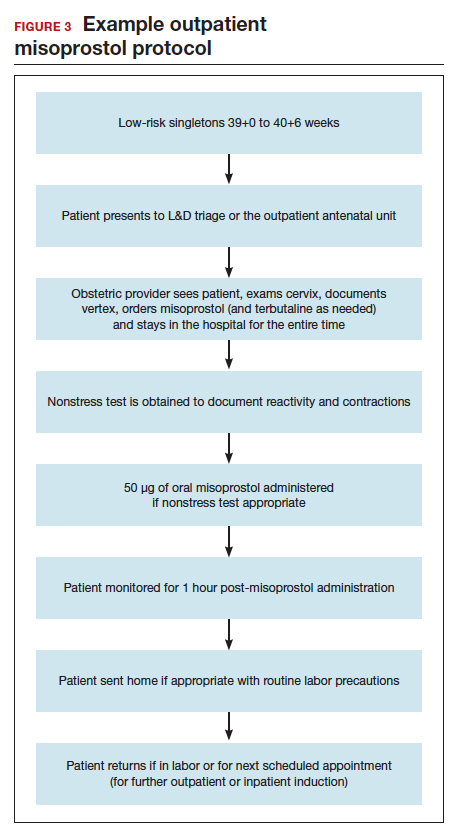
Our hospital has been conducting outpatient cervical ripening using vaginal misoprostol for more than 15 years without any known adverse safety concerns (FIGURE 3). Women with a low-risk, singleton pregnancy between 39+0 and 40+6 weeks are potential candidates for outpatient ripening. The majority of outpatient inductions are done electively without any medical indication. Women with stable, minor risk factors (such as diet-controlled gestational diabetes) also may be candidates at their clinician’s discretion. Patients are monitored either in our L&D triage area or in our outpatient antenatal unit; both units are in the same building. One clinician offers outpatient misoprostol in the office, across the street from L&D. We allow for clinician flexibility after administration. Some clinicians do 1 or 2 doses of outpatient cervical ripening in a day prior to a scheduled inpatient induction the next day. Some do multiple daily doses over the course of a week.
Conclusion
While the data continue to be limited, we strongly believe there is sufficient quality evidence from a safety and efficacy perspective to support implementation and evaluation of outpatient cervical ripening protocols for low-risk pregnancies. In the setting of renewed commitments to reducing suboptimal health care costs and utilization as well as increasing patient satisfaction and control in their birthing experiences, we posit it is the responsibility of obstetricians, L&D leadership, and health care institutions to explore the implementation of outpatient cervical ripening for appropriate candidates in their settings.