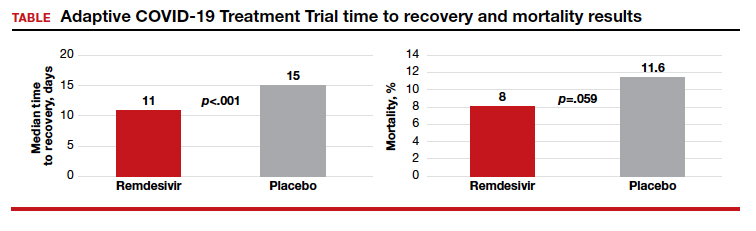
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...