Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
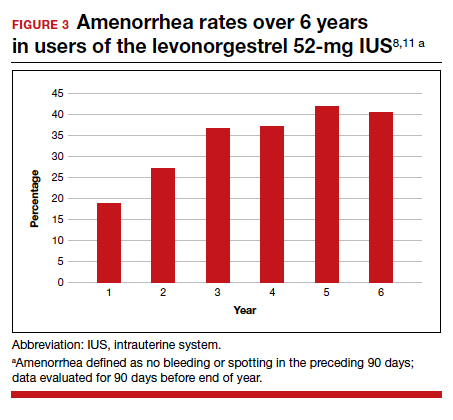
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.
Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.