Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
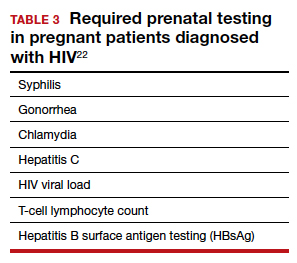
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...