LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
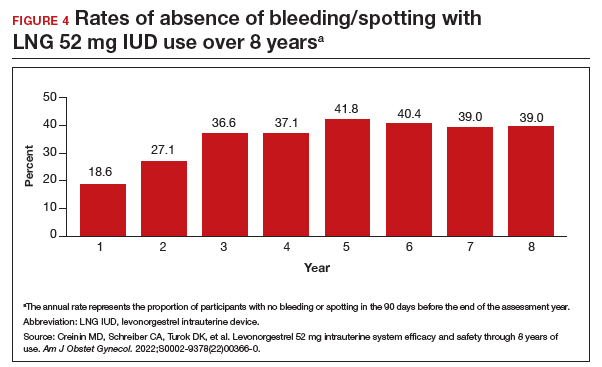
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●
As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.