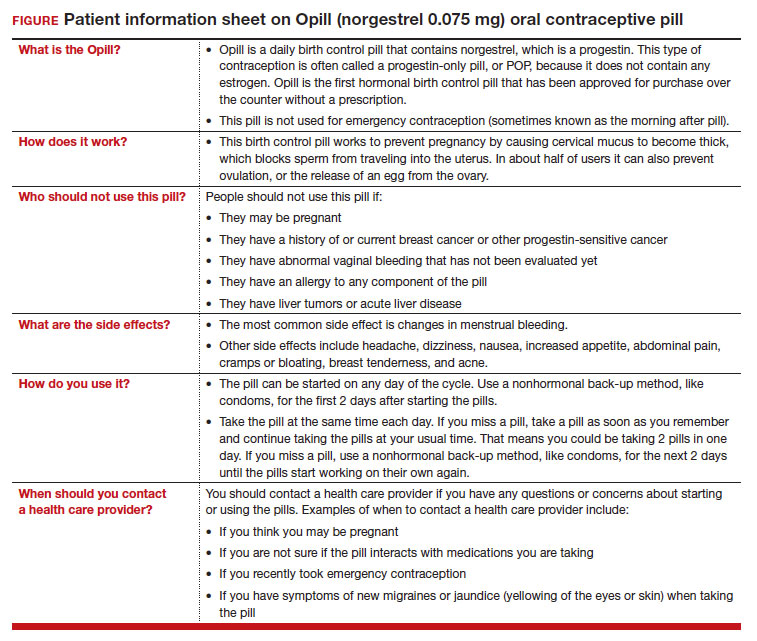
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.
How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...