Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
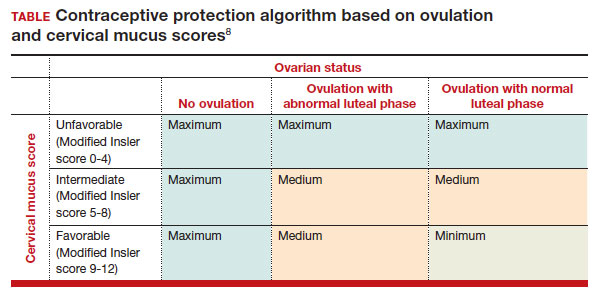
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8
In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...