Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
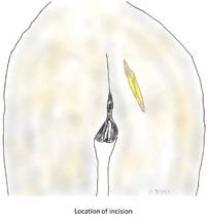
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.