The panel discussed whether the exclusion of patients with a prior clinical breast intervention should be specified in the indication, and agreed that it should, given the pivotal study design. Additional studies would be needed to remove the restriction, the panel said.
Some panelists expressed concern over whether somo-v use on biopsy patients, for instance, might increase the device’s false-positive rate, given surgical scars’ similarity to malignancies on ultrasound.
Nevertheless, a majority did not seem overly concerned about potential off-label use on patients with prior interventions. "It seems to me that that’s not a big problem," said panel member Dr. Daniel Kopans, professor of radiology at Harvard Medical School, Boston. "If FDA approves the technology even with these limitations, those of us in breast imaging will use it as we see fit. ... I don’t think that [the indication] is a huge limitation."
Operator Training Concerns
The panel also discussed the sponsor’s proposed physician training program, concluding that it is adequate. However, after the panel voted on safety and effectiveness, some members debated whether potential use of the system by nonimaging specialists could adversely impact outcomes and whether controls were needed to restrict use to accredited radiologists.
According to the company, somo-v’s automation is intended to "reduce operator dependence," and the firm claims that "any medical staff member can be trained to operate the ABUS device consistently and reproducibly." But some panel members cautioned that the simplicity could lead to harm in clinical practice.
"Potentially, you could be in a situation where the mammogram is done by breast-imaging specialists, but the ultrasound is done by nonimaging specialists, which could significantly affect results," said panelist Dr. Robert Faulk, a diagnostic radiologist in private practice in Omaha, Neb.
Dr. Kopans agreed, citing the Mammography Quality Standards Act’s radiologist accreditation training and urging that "similar constraints" are needed for somo-v.
But in response, Janine Morris, the FDA’s acting radiologic devices division director, reminded the panelists that the FDA "doesn’t regulate [the] practice of medicine."
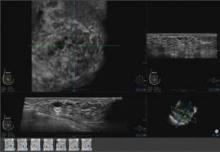
Somo-v relies on proprietary hardware and software, and incorporates a scan head that automatically acquires wide field-of-view breast images while the patient lies supine on a standard exam table.
The images are transferred to the system’s somo-VIEWer 3D workstation, which displays the images in a 3D reconstructed coronal view, as well as standard ultrasound formats, including transverse, radial, multislice, sagittal and antiradial views.
Elsevier Global Medical News and "The Gray Sheet" are owned by Elsevier.