Managing psoriasis during pregnancy includes consideration for quality of life and medication safety issues, according to Dr. Alan Menter.
"Treatment of pregnant women with psoriasis should take into consideration the benefit of the therapy to her and the safety of her fetus. The full spectrum of safe and effective therapies during pregnancy needs to be reviewed," Dr. Menter said at the Women’s & Pediatric Dermatology Seminar, sponsored by the Skin Disease Education Foundation (SDEF).
"Most people present with psoriasis before age 40 years. The disease affects women and men equally, so many women with psoriasis are of reproductive age and are considering pregnancy," said Dr. Menter, chairman of the dermatology division at Baylor University Medical Center, Dallas.
"Maintaining quality of life is particularly important for females with psoriasis who are considering becoming pregnant, as well as during pregnancy and while breastfeeding."
Treatment Options
Patients with mild psoriasis can be treated safely with topical agents during pregnancy, most commonly with steroid and vitamin D preparations. For women with psoriasis unresponsive to topicals, a course of phototherapy with narrow-band UVB is safe and effective before conception, during pregnancy, and during the lactation period, Dr. Menter said.
Methotrexate, which should be stopped 3 months before conceiving, and the retinoid acitretin (Soriatane), which is contraindicated in females of childbearing potential, are pregnancy category X drugs and are not options during pregnancy.
As for cyclosporine, more than 20 years of pregnancy registry data on organ transplant recipients treated with the drug – who are on higher doses than used in psoriasis – indicate that cyclosporine is a "viable drug to be used during pregnancy if absolutely necessary," he said.
For moderate to severe psoriasis, "cyclosporine works very well at relatively low dosages, but has to be discontinued before the baby is born because it is excreted in breast milk," he added. A potential adverse effect of cyclosporine is that it may aggravate hypertension of pregnancy, and, in a small percentage of patients, it produces a pregnancy that is 3-4 weeks shorter than normal.
The biologic treatments etanercept (Enbrel), adalimumab (Humira), infliximab (Remicade), and ustekinumab (Stelara), which are all category B drugs, could interfere with normal embryonic development and affect fetal and newborn immunity. However, there are numerous pregnancy registries for both psoriasis and other inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis, which show that biologics have had no long-term implications for the normal development of the infant immune system.
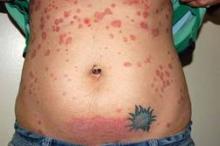
Psoriasis improves spontaneously in about 60% of women during pregnancy, although they are at an increased risk for postpartum flares. After delivery, "psoriasis can flare up very quickly and can spread from head to toe within days or weeks ... [and is] sometimes a lot worse than what they had previously," he said.
Patients should be advised to alert their physicians at the first sign of a flare after delivery. Phototherapy and topical treatments are options, but cyclosporine should not be used in breastfeeding patients. There is almost no excretion of the four injectable biologics into breast milk, which can thus be considered safe in lactating women.
Case Studies
Among the cases he presented at the conference was a 26-year-old female with a 5-year history of severe psoriasis who started treatment with cyclosporine. At 12 months, she was 90% clear and the dose was tapered. She became pregnant at 16 weeks. Cyclosporine was discontinued at 27 weeks gestation, and she remained relatively clear for the rest of her pregnancy and delivered a healthy baby at term.
She had a severe postpartum flare, with only a moderate response obtained with the reintroduction of cyclosporine after she stopped breastfeeding. She was then started on a 12-week course of the biologic agent alefacept and UVB treatment, which produced a moderate response. During the second month of alefacept treatment, she became pregnant again. The moderate response achieved with the alefacept was maintained throughout the pregnancy, even after the 12-week course of alefacept was stopped. She delivered a normal, healthy baby.
The same patient was started on infliximab for a significant flare of psoriasis a month after her second delivery. She was 95% clear after three induction infusions and was maintained on infliximab for 5 years. She became pregnant again during treatment with infliximab and had a healthy baby, with a normal delivery. She remains 90% clear to date on infliximab at a dose of 7.5 mg/kg every 8 weeks.