Hepatitis B virus (HBV) infects between 128,000 and 320,000 people each year in the United States. Approximately 1.25 million Americans have chronic infection. The lifetime risk of acquiring the disease is estimated at 5%.7 More than 5,000 people die of HBV-related liver disease annually, and HBV infection is the second leading cause of cancer worldwide.
Measles, mumps, and rubella (MMR) cases declined greatly in the 20th century, thanks to widespread vaccination. By 2001, the number of cases reported annually had declined to 116 for measles (from an all-time high of more than 500,000), to 266 for mumps, and to 23 for rubella.8,9 Although the vaccines for these 3 viruses are extremely effective, occasional outbreaks of all 3 illnesses are still reported in the United States. Measles outbreaks occur because the disease is highly contagious, with an attack rate of 90% or higher among unvaccinated household contacts.
Varicella. Before vaccination became available, roughly 4 million cases of varicella zoster virus infection occurred each year in the United States. Although the virus usually causes relatively benign chickenpox, death is a possibility. From 1990 to 1994, for example, before the varicella vaccination program, 11,000 hospitalizations and 100 deaths were attributed to varicella disease each year.10 Most of those who died were previously healthy.
During 2002, 9 fatal cases of varicella in adults and children were reported.10 That figure likely represents only a partial accounting of varicella-related deaths. According to National Center for Health Statistics data for 2000, varicella was listed as the primary cause of death on 44 death certificates in 23 states and the District of Columbia, although only 9 (20%) varicella-related deaths were reported to the CDC.10
Although it is roughly 7 times more expensive than a flu shot (at approximately $46 per dose), the new intranasal vaccine, FluMist, has appealing qualities—especially for people who spurn vaccination to avoid the needle. FluMist (MedImmune Inc, Gaithersburg, Md) is a cold-adapted, live, trivalent vaccine approved June 17, 2003, by the US Food and Drug Administration for ages 5 to 49 years. It is available for the 2003–2004 influenza season.
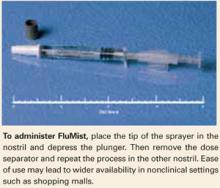
Easy to administer. Each prefilled sprayer contains a 0.5-mL dose, which clinicians administer by placing the Teflon tip into the patient’s nose and depressing the plunger. (A dose separator ensures that 0.25 mL is delivered into each nostril.) Because of its simplicity, the vaccine ultimately could be available in nonclinical settings such as shopping malls, boosting the number of persons who get immunized each year.
FluMist induces intranasal immunoglobulin A that is specific for each of the 3 influenza strains targeted this season by the US Public Health Service. This is important because influenza virus enters the body through the nose. (The shots do not induce intranasal immunoglobulin.)
How effective is FluMist? The estimated efficacy of live-attenuated vaccine in healthy adults exposed to wild-type influenza A and B viruses was 85% in 1 study, but ranged as high as 100% in a meta-analysis.19,20
When FluMist was tested in a randomized, double-blind, placebo-controlled trial, recipients were as likely as controls to experience 1 or more febrile illnesses during peak outbreak periods. However, they had significantly fewer severe febrile illnesses and febrile upper respiratory tract illnesses. They also missed fewer work days and required fewer visits to a healthcare provider.21
Contraindications include pregnancy. Because its effects during pregnancy are unknown, FluMist should not be given to gravidas.
Further, persons with a history of Guillain-Barré syndrome or hypersensitivity to eggs or egg products should not receive the vaccine. Nor should FluMist be given to those with known or suspected immune deficiency diseases or immuno-suppression, or whose immune status may be depressed due to therapies such as systemic corticosteroids, antimetabolites, alkylating drugs, and radiation.22
Precautions. Vaccinated persons may shed live virus and should avoid close contact with immunocompromised people for 21 days or more.
The effects of administering FluMist at the same time as other vaccines have not been studied. For this reason, it is best given alone.
Adverse reactions include cough, runny nose, sore throat, chills, tiredness or weakness, nasal congestion, rhinitis, and sinusitis.
Vaccination during pregnancy: Benefits versus risks
As the American College of Obstetricians and Gynecologists (ACOG) notes in a committee opinion,11 preconception vaccination “to prevent disease in the offspring, when practical, is preferred to vaccination of pregnant women.” However, in pregnancy, the benefits of immunization usually outweigh the risk of adverse events when:
- the likelihood of exposure to disease is high,
- infection would pose a risk to the mother or fetus, and
- the vaccine is unlikely to cause illness or injury.12