REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
3 HPV vaccine, soon to be in our offices
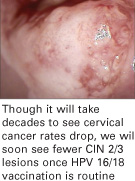
Vaccines will stop CIN 2/3 and cancer
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27.
The HPV 16 vaccine provided 100% protection against development of HPV-16-related CIN 2/3 during an average of 3.5 years of follow-up.
This preliminary study to the Quadrivalent HPV 6, 11, 16, 18 trial on an HPV 16 virus-like particle (VLP) vaccine reached an average of 3.5 years of follow-up. CIN 2/3 developed in 12 of the 750 women receiving placebo, in contrast to none of the 755 vaccine recipients. Persistent HPV 16 infections were defined as testing positive for type-specific HPV 16 on 2 or more visits, with the caveat that women testing positive on the last visit were considered persistent because they would have no further follow-up to determine that status. As a result, some women with a positive test only on the last visit were included as “persisters,” perhaps explaining why the efficacy in preventing persistent HPV 16 in the vaccine recipients was only 94%. Single-test positives can be transient infections, vaginal contamination with infected cells from a partner during recent intercourse, or early persistent infections. Although antibody titers to HPV 16 in vaccine recipients waned over time, they still exceeded titers in placebo recipients who already had natural immunity to HPV 16.
Benefit of the HPV 16 vaccine was also seen for women already HPV-16 positive at enrollment, but only if they were seronegative for HPV 16. It is possible that, if an immune response has not yet been mounted, the vaccine may still have a positive effect for women already HPV-16 infected.
Who will be vaccinated?
Although the primary target group for the HPV vaccine will be children before natural exposure can occur after the onset of sexual activity, many women already sexually active will likely want to be vaccinated.
It is this “catch-up” group that will challenge the OBGyn to become familiar with and to provide the HPV vaccine when it becomes available, likely later this year.
Quadrivalent vaccine 100% effective
Skjeldstad FE, Koutsky LA, for the Merck Phase 3 HPV Vaccine Steering Committee (Future II). Phase II trial of prophylactic quadrivalent HPV 6, 11, 16, 18 L1 virus-like particle (VLP) vaccine; prevention of cervical intraepithelial neoplasia (CIN) 2/3 including adeno- and squamous cell carcinoma in situ (CIS). Presented at: Infectious Diseases Society of America, Late Breaker Session 66, LB–8A; October 7, 2005; San Francisco, Calif.
The Quadrivalent HPV 6, 11, 16, 18 vaccine provided 100% protection against persistent HPV 16/18 and HPV 16/18-related CIN 2/3.
This trial became center stage in the world media in early October 2005, with headlines such as “First anti-cancer vaccine 100% effective.” The results are truly astounding, as there were no CIN 2/3 cases in the Per Protocol group, among the 5,301 women vaccinated, in contrast to 21 cases in the 5,258 women who received the placebo (TABLE 1).
In the group most likely to mirror a typical vaccinated population, only 1 case of HPV-16/18-positive CIN 2/3 occurred in the 5,736 vaccinated women, some of whom were already positive for 1 or more HPV types, or had serologic evidence of prior type-specific infection, or received fewer than the 3 recommended doses. In contrast, 36 HPV-16/18 CIN 2/3 occurred in the 5,766 women who received placebo.
TABLE 1
Efficacy of quadrivalent HPV 6, 11, 16, 18 vaccine in preventing CIN 2/3
| VACCINE GROUPS | PLACEBO GROUPS | EFFICACY | |||||
|---|---|---|---|---|---|---|---|
| NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | NUMBER OF WOMEN | NUMBER OF CIN 2/3 CASES | RATE | % | |
| Per protocol | 5,301 | 0 | 0.0 | 5,258 | 21 | 0.3 | 100 |
| Modified intention to treat | 5,301 | 1 | <0.1 | 5,766 | 36 | 0.3 | 97 |
| Source: Skjeldstad et al | |||||||
No warts, either
Subsequent analysis revealed similar protection from HPV 6 or 11 genital warts.
No serious adverse events were recorded in the entire trial.
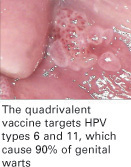
Because HPV 16 and 18 together cause approximately 70% of all cervical cancers, and HPV 6 and 11 cause 90% of genital warts, these results are surely something about which to rejoice!
Gardasil and Cervarix vaccines
Now the challenge will be in getting the population vaccinated. Merck is expected to have its Gardasil Quadrivalent vaccine on the market mid- to late 2006. GlaxoSmithKline expects to put Cervarix Bivalent HPV 16, 18 vaccine on the market sometime in 2007.


