The most compelling prediction of the risk of CIN 3+ in cases of either single (incident) or persistent high-risk HPV detection comes from a 13-year follow-up study out of Denmark. More than 8,600 Danish women, 20 to 29 years old at enrollment, were tested twice, 2 years apart, for HPV by the Hybrid Capture 2 High-Risk (HC2 HR) HPV DNA Test (Qiagen) and by line-blot assays for type-specific HPV.
Subjects were followed subsequently with routine screening; data on their overall long-term risk of CIN 3+ was obtained from the national Danish Pathology Data Bank. Among those who tested positive for HPV 16, absolute risk of CIN 3+ within 12 years of incident detection was 26.7%; for HPV 18, it was 19.1%—both percentages markedly higher than the 6% risk for women who tested positive for a panel of 10 other high-risk HPV types (also not including HPV 31, 33).
FIGURE 2 Over time, risk of CIN 3+ rises in all subpopulations infected by high-risk HPV
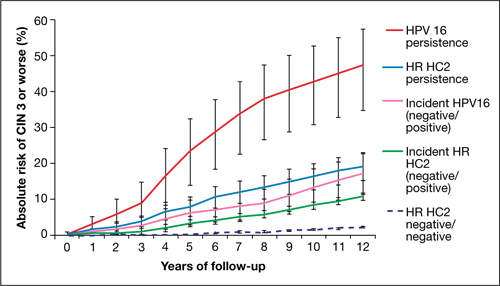
Shown is the absolute risk for CIN 3+ during follow-up among women who have HPV 16 persistence (red line), Hybrid Capture 2 High-Risk HPV DNA Test (HC2 HR) persistence for a panel of 13 high-risk HPV types (blue), incident or newly detected HPV 16 infection (i.e., HPV 16-negative at first examination and HPV 16-positive at second examination) (pink), and women with an incident high-risk HPV infection (i.e., negative for high-risk HPV by HC2 HR at first examination and positive at second examination) (green). For comparison, the dashed purple line shows the nearly flat absolute risk of CIN 3+ for women who are negative for high-risk HPV by HC2 HR at both examinations.
Source: Kjaer et al, 2010. Reproduced by permission of the publisher, Oxford University Press.
Risk associated with HPV 16. The most successfully persistent viral type was 16; just under 30% of subjects who were HPV 16+ on the first test remained HPV 16+ on the second test 2 years later. For this 30% subset, the risk of CIN 3+ was 8.9% at 3 years; 23.8% at 5 years; and an astonishing 47.4% at 12 years (FIGURE 2). In other words, almost half of subjects who had persistent HPV 16 in their 20s developed CIN 3 or cancer within 12 years. In contrast, the absolute risk for developing CIN 3+ within 12 years of a single negative HPV test was only 3%.
HPV 18. Viral type 18 was nearly as likely to persist as HPV 16; when persistent, it resulted in CIN 3+ in just under 30% of subjects within 12 years and tended to result in CIN 3+ that was detected later.
The long-term risk of CIN 3+ and cervical cancer that comes with testing positive for HPV 16 or 18 is very high—particularly when either of these viral types are detected twice over a 2-year period. Even one-time detection of either of these types in the context of a Pap–/HPV+ co-test result is important enough to refer to colposcopy.
Using HPV 16, 18 testing to improve the management of Pap–/HPV+ women
Two more HPV tests are now FDA-approved for clinical use
In the 2006 Update on Cervical Disease [available in the archive at www.obgmanagement.com], I noted the importance of HPV 16, 18 in cervical carcinogenesis and observed that a test for these two viral types was on the horizon. It wasn’t until 2009 approval of the Cervista HPV DNA HR Test (Hologic), however, that such a test became available.
In 2011, two more tests were approved:
- cobas 4800 HPV DNA
- Aptima mRNA HPV.
Like Cervista, the cobas 4800 (Roche) is FDA-approved to detect HPV DNA types 16 and 18 individually; Gen-Probe’s Aptima HPV test is the first HPV E6,E7 mRNA test approved in the US; the company plans to add an HPV 16, 18 mRNA test. The addition of one, perhaps two, new HPV 16, 18 genotyping tests expands the opportunity for you to improve the management of Pap–/HPV+ patients.
Let’s look at new data that support testing of Pap–/HPV+ women for HPV 16, 18.
Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136(4):578–586.
Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12(9):880–890.


