From the Editor
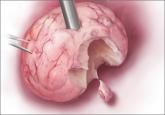
Benefits and pitfalls of open power morcellation of uterine fibroids
The current practice of open power morcellation is being scrutinized by those within and outside of the ObGyn community. We need to re-examine our...
Deborah Reale, Managing Editor

Hospitals suspend use of power morcellators until further notice
Click here to access articles, videos, and audiocasts in the Morcellation Topic Collection
On April 17, 2014, the US Food and Drug Administration (FDA) issued a Safety Communication discouraging the use of laparoscopic power morcellation in hysterectomy and myomectomy for uterine fibroids.
Based on an FDA analysis of current data, “… it is estimated that 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma. If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient’s likelihood of long-term survival.”1
FDA recommendations
The FDA posted the following recommendations for health-care providers1:
Although many women choose laparoscopic hysterectomy or myomectomy because of the associated benefits, there are other treatments available, including vaginal or abdominal hysterectomy and myomectomy; laparoscopic hysterectomy or myomectomy without morcellation; minilaparotomy; uterine artery embolization; high-intensity focused ultrasound; and drug therapy.
FDA actions
To reduce the risk of inadvertent spread of unsuspected cancer to the abdomen and pelvis, the FDA has instructed manufacturers of power morcellators used during laparoscopic hysterectomy and myomectomy to immediately review labeling for accurate risk information.
A to-be-convened public meeting of the FDA’s Obstetrics and Gynecological Medical Device Advisory Committee will discuss1:
The FDA will continue to review adverse event reports and peer-reviewed literature, as well as patient information and evidence from health-care providers, gynecologic and surgical professional societies, and medical device manufacturers.
Adverse events should promptly be reported to the FDA by filing a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
Institution reaction
Brigham and Women's Hospital had banned the use of open power morcellation on March 31, 2014, allowing for the use of power morcellators within a containment system. In light of the FDA notice that discourages the use of power morcellators during hysterectomy or myomectomy for the treatment of uterine fibroids, however, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s advised surgical staff to "immediately suspend use of power morcellators in all cases until further notice."
Massachusetts General, which also had placed restrictions on the use of power morcellators prior to the FDA communication, suspended the use of power morcellation.
“I have asked our doctors to stop the procedure immediately until more information is available,’’ Dr. Isaac Schiff, Massachusetts General’s chief of obstetrics and gynecology, told the Boston Globe.

The current practice of open power morcellation is being scrutinized by those within and outside of the ObGyn community. We need to re-examine our...
The most promising alternative to open power morcellation is morcellation in a bag, described here
Letters from Readers
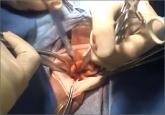
Ceana Nezhat, MD, and Erica Dun, MD, show how they perform enclosed vaginal morcellation
This technique is K. Anthony Shibley, MD's, short-term surgical solution to performing closed power morcellation. His patented pneumoperitoneum...
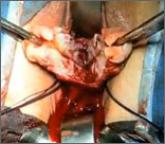
Dr. Shibley discusses a novel strategy he has developed to address the problem of tissue dispersion during open power morcellation
