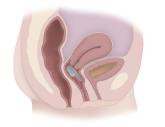
A vaginally inserted device that uses a balloon inflated by the patient to occlude the rectum has been cleared for marketing as a treatment for fecal incontinence in adult women, the Food and Drug Administration announced on Feb. 12.
The device is indicated for women aged 18-75 years who have had at least four episodes of fecal incontinence (FI) in a 2-week period, according to the FDA’s statement. The device includes a vaginal insert, placed in the same area as a tampon, and a pump that inflates and deflates a balloon on the insert. When inflated by the woman, the balloon “exerts pressure through the vaginal wall onto the rectal area, thereby reducing the number of FI episodes,” the statement said. The first time it is used, the clinician fits the device and inflates the balloon; subsequently, a patient deflates and inflates the device on her own, as needed. It will be marketed as the Eclipse System, by Pelvalon.
In the statement, Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, pointed out that treatment options for fecal incontinence currently include drugs, dietary changes, exercise, and surgery, and that the Eclipse System “provides an additional treatment option for women who suffer from this condition.”
Like many devices, this device did not go through the full approval process but was reviewed by the FDA through the “de novo classification process,” a pathway for making available some devices that have low to moderate risk and are not considered substantially equivalent to another available device.
The FDA reviewed nonclinical testing data and the main study that evaluated the device in 61 women with FI. One month after they started using the device, the number of FI episodes dropped by 50% in almost 80% of the women in the trial, according to the FDA statement. Pelvic cramping and discomfort; pelvic pain; vaginal abrasion, redness, or discharge; and urinary incontinence were among the adverse events associated with the device; all adverse events were mild or moderate. The LIFE study is scheduled to be published in the March 2015 issue of Obstetrics and Gynecology.
In a statement, the company said that it plans to release the Eclipse System later in 2015.
emechcatie@frontlinemedcom.com