Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.
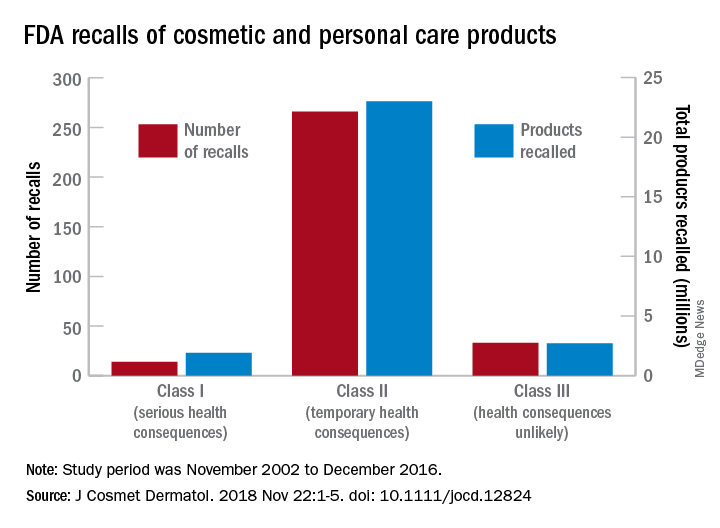
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.