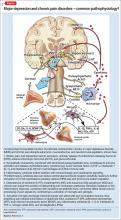
Likewise, the mPFC is a key component of the default mode network (DMN), a functional network also comprising the posterior cingulate cortex and hippocampus. DMN performs a diverse set of activities, including self-reflection, daydreaming, reminiscing, planning, processing of social information, and creative thinking. Negative neuroplastic changes in the DMN are a common finding in MDD and chronic pain, and might be associated with a tendency toward rumination and catastrophizing—key clinical manifestations of MDD and chronic pain—and linked with pervasive negative affect and sleep disturbance.4,32
Furthermore, functional and structural changes in the amygdala and hippocampus have been described in MDD, fibromyalgia, and neuropathic pain.4 Dysfunction of these limbic formations may be a contributing factor in the disruption of neuroendocrine, autonomic, and immune function, which could further contribute to aggravated mood and pain symptoms.4,17,40
Consequently, excessive hypothalamic-pituitary-adrenal axis and sympathetic activation, combined with elevation of proinflammatory cytokine production and release, likely plays a role in the pathophysiology of MDD and chronic pain disorders.4,17,40 Moreover, at cellular, subcellular, and molecular levels, chronic pain and MDD are associated with:
- perturbed neuron-glia relationships
- altered glutamatergic, GABA, glycine, substance-P, opioid, 5-HT, norepinephrine, and dopamine signaling
- dysfunction of intracellular signaling cascades and neurotrophic signaling.4,20,30,31,38
The Figure that describes how homeostatic function of prefrontal cortical-limbic circuitry is compromised in MDD and chronic pain—thus disrupting autonomic, neuroendocrine, and neuroimmune regulation.
Disturbance in monoamine signaling in chronic pain and MDD might give rise to profound anhedonia, cognitive impairment, anxiety, insomnia, sensitivity to stress, and inadequate functioning of descending pain-regulatory pathways, which primarily use norepinephrine and 5-HT.4,9,20,30,31,38 Using pharmacotherapeutic agents that successfully modulate monoamines, therefore, might ameliorate the function of brain networks innervated by neurotransmitter systems involved in the regulation of pain, mood, cognition, stress response, and sleep. Notably, the same monoamines serve as transmitters in descending pain pathways.
In summary, convergent evidence indicates that MDD and chronic pain states amplify each other, thus contributing to treatment resistance in both disorders.
On the bright side, timely and effective treatment of MDD might optimize the chance of remission and minimize the risk of enduring structural brain changes in MDD and chronic pain.1,4,31,32 The obverse is also true: Emphasizing the importance of the resolution of painful symptoms in the context of MDD, a study reported a significantly greater remission rate of 36.2% in those who had >50% reduction of pain on a visual analogue scale following treatment with a serotonin-norepinephrine reuptake inhibitor, compared with a 17.8% remission rate in persons who experienced <50% pain reduction on the scale.3
Editors’ note: In Part 2 of this article (March 2016), the authors review pharmacotherapeutic and non-drug strategies for managing comorbid chronic pain conditions and MDD.