Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
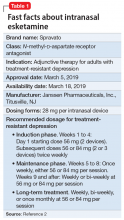
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal esketamine (brand name: Spravato) (Table 12) following the evaluation of its efficacy through short-term clinical trials and a longer-term maintenance-of-effect trial. Intranasal esketamine is indicated, in conjunction with an oral antidepressant, for adult patients with TRD.2 Esketamine is a CIII controlled substance, and concerns about abuse, misuse, and diversion have been taken into account within the Risk Evaluation and Mitigation Strategy (REMS) drug safety program. The agent is only available through a restricted distribution—the REMS will mandate that REMS certified pharmacies dispense directly to a REMS certified treatment program. Intranasal esketamine will not be sampled or dispensed directly to patients.
How it works
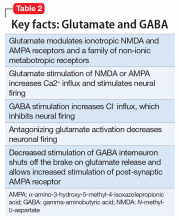
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and a family of non-ionic metabotropic receptors, or mGluRs. Glutamate stimulation of NMDA or AMPA receptors increases Ca2+ ion influx and enhances neural firing. Conversely, GABA stimulation increases Cl– ion influx, which inhibits neural firing. Antagonizing glutamate receptors inhibits neural firing. N-methyl-d-aspartate receptors localized on the GABA interneuron modulate GABAergic activity. Antagonism of the NMDA receptor on GABA interneurons decreases GABA activity. Decreased activity of the GABA interneuron promotes intrasynaptic glutamate release and enhances glutamate stimulation of postsynaptic AMPA receptors. Glutamate stimulation of AMPA receptors then stimulates a cascade of intrasynaptic signaling that promotes the release of brain-derived neurotrophic factor (BDNF) and increased production of neuronal membrane proteins with subsequent neural plasticity.
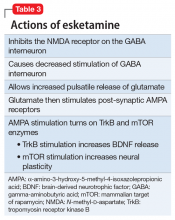
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...