Approved by the FDA on October 11, 2019, asenapine transdermal system (Secuado, manufactured by Hisamitsu Pharmaceutical Co., Inc. and distributed by Noven Therapeutics) is the first “patch” approved by the FDA for the treatment of adults with schizophrenia (Table 1).1-3 Asenapine is a second-generation antipsychotic that was previously available only as a sublingual formulation (Saphris, Allergan [now AbbVie] under license from Merck Sharp & Dohme B.V.).4-7 Asenapine’s reformulation potentially simplifies using this antipsychotic by reducing the dosing frequency from twice daily (recommended for the sublingual tablet) to once daily (recommended for the patch). The food and drink restrictions posed by the sublingual formulation are eliminated. Also avoided are dysgeusia (bad taste) and hypoesthesia of the tongue (numbing). Asenapine transdermal system offers a different method to manage schizophrenia, and this novel method of administration through the skin is worth considering.8
The asenapine transdermal system is available in 3 patch sizes: 20, 30, and 40 cm2, which deliver 3.8, 5.7, and 7.6 mg/24 hours of asenapine, respectively.3 Based on the average exposure (area under the plasma concentration curve [AUC]) of asenapine, 3.8 mg/24 hours corresponds to 5 mg twice daily of sublingual asenapine, and 7.6 mg/24 hours corresponds to 10 mg twice daily of sublingual asenapine.3 The “in-between” dose strength of 5.7 mg/24 hours would correspond to exposure to a total of 15 mg/d of sublingual asenapine. The recommended starting dose for asenapine transdermal system is 3.8 mg/24 hours. The dosage may be increased to 5.7 mg/24 hours or 7.6 mg/24 hours, as needed, after 1 week. The safety of doses above 7.6 mg/24 hours has not been evaluated in clinical studies. Asenapine transdermal system is applied once daily and should be worn for 24 hours only, with only 1 patch at any time. Application sites include the upper arm, upper back, abdomen, and hip. A different application site of clean, dry, intact skin should be selected each time a new patch is applied. Although showering is permitted, the use of asenapine transdermal system during swimming or taking a bath has not been evaluated. Of note, prolonged application of heat over an asenapine transdermal system increases plasma concentrations of asenapine, and thus application of external heat sources (eg, heating pads) over the patch should be avoided.
How it works
Product labeling notes that asenapine is an atypical antipsychotic, and that its efficacy in schizophrenia could be mediated through a combination of antagonist activity at dopamine D2 and serotonin 5-HT2A receptors.3 The pharmacodynamic profile of asenapine is complex5 and receptor-binding assays performed using cloned human serotonin, norepinephrine, dopamine, histamine, and muscarinic receptors demonstrated picomolar affinity (extremely high) for 5-HT2C and 5-HT2A receptors, subnanomolar affinity (very high) for 5-HT7, 5-HT2B, 5-HT6, and D3 receptors, and nanomolar affinity (high) for D2 receptors, as well as histamine H1, D4, a1-adrenergic, a2-adrenergic, D1, 5-HT5, 5-HT1A, 5-HT1B, and histamine H2 receptors. Activity of asenapine is that of antagonism at these receptors. Asenapine has no appreciable affinity for muscarinic cholinergic receptors.
The asenapine receptor-binding “fingerprint” differs from that of other antipsychotics. Some of these receptor affinities are of special interest in terms of potential efficacy for pro-cognitive effects and amelioration of abnormal mood.5,9 In terms of tolerability, a relative absence of affinity to muscarinic receptors would predict a low risk for anticholinergic adverse effects, but antagonism at histamine H1 and at a1-adrenergic receptors, either alone or in combination, may cause sedation, and blockade of H1 receptors would also predict weight gain.9 Antagonism of a1-adrenergic receptors can be associated with orthostatic hypotension and neurally mediated reflex bradycardia.9
Clinical pharmacokinetics
Three open-label, randomized, phase 1 studies were conducted to assess the relative bioavailability of asenapine transdermal system vs sublingual asenapine.10 These included single- and multiple-dose studies and clinical trials that examined the effects of different application sites and ethnic groups, and the effect of external heat on medication absorption. Studies were conducted in healthy individuals, except for the multiple-dose study, which was performed in adults with schizophrenia. The AUC for asenapine transdermal system was within the range of that of equivalent doses of sublingual asenapine, but peak exposure (maximum concentration) was significantly lower. As already noted, the AUC of the asenapine patch for 3.8 mg/24 hours and 7.6 mg/24 hours corresponds to sublingual asenapine 5 mg and 10 mg twice daily, respectively. Maximum asenapine concentrations are typically reached between 12 and 24 hours, with sustained concentrations during the 24-hour wear time.3 On average, approximately 60% of the available asenapine is released from the transdermal system over 24 hours. Steady-state plasma concentrations for asenapine transdermal system were achieved approximately 72 hours after the first application and, in contrast to sublingual asenapine, the peak-trough fluctuations were small (peak-to-trough ratio is 1.5 for asenapine transdermal system compared with >3 for sublingual asenapine). Dose-proportionality at steady state was evident for asenapine transdermal system. This is in contrast to sublingual asenapine, where exposure increases 1.7-fold with a 2-fold increase in dose.4,5 Following patch removal, the apparent elimination half-life is approximately 30 hours.3 The pharmacokinetics of the patch did not vary with regards to the application site (upper arm, upper back, abdomen, or hip area), and the pharmacokinetic profile was similar across the ethnic groups that participated in the study. Direct exposure to external heat did increase both the rate and extent of absorption, so external heat sources should be avoided.3
Efficacy
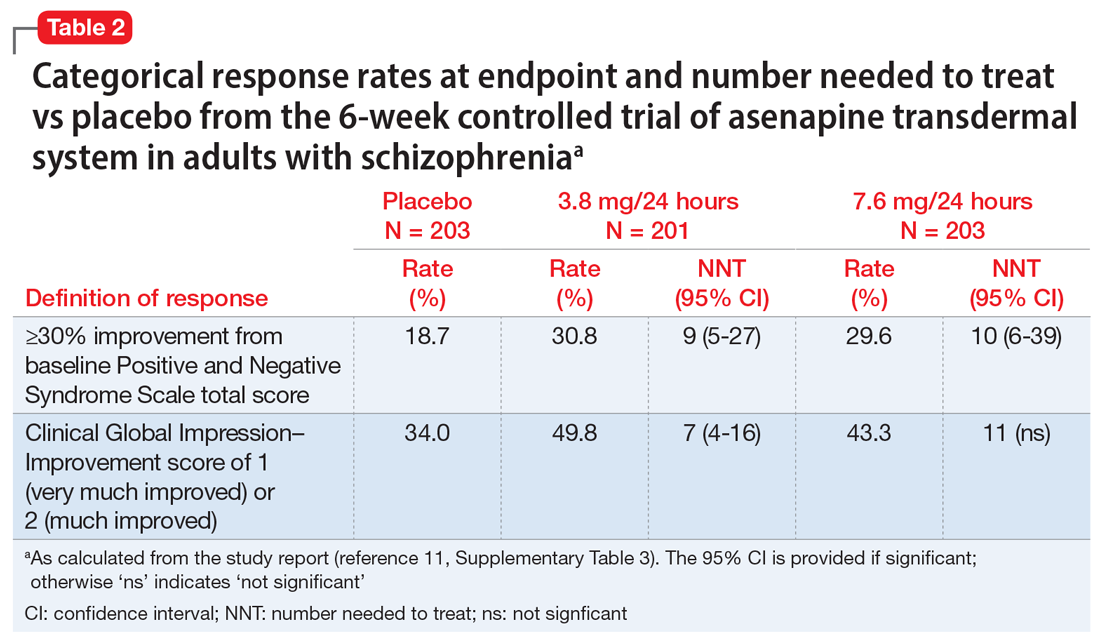
The efficacy profile for asenapine transdermal system would be expected to mirror that for sublingual asenapine.6,7 In addition to data supporting the use of asenapine as administered sublingually, a phase 3 study specifically assessed efficacy and safety of asenapine transdermal system in adults with schizophrenia.11,12 This study was conducted in the United States and 4 other countries at a total of 59 study sites, and 616 patients with acutely exacerbated schizophrenia were enrolled. After a 3- to 14-day screening/single-blind run-in washout period, participants entered a 6-week inpatient double-blind period. Randomization was 1:1:1 to asenapine transdermal system 3.8 mg/24 hours, 7.6 mg/24 hours, or a placebo patch. Each of the patch doses demonstrated significant improvement vs placebo at Week 6 for the primary (change in Positive and Negative Syndrome Scale [PANSS] total score) and key secondary (change in Clinical Global Impression-Severity of Illness) endpoints. Response at endpoint, as defined by a ≥30% improvement from baseline PANSS total score, or by a Clinical Global Impression–Improvement score of 1 (very much improved) or 2 (much improved), was also assessed. For either definition of response, both doses of asenapine transdermal system were superior to placebo, with number needed to treat (NNT) (Box) values <10 for the 3.8 mg/24 hours dose (Table 2). These effect sizes are similar to what is known about sublingual asenapine as determined in a meta-analysis performed by the manufacturer and using individual patient data.13
Box
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we reviewing a result that may be statistically significant but irrelevant for day-today patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine—can help answer these questions. NNT helps us gauge effect size or clinical significance. It is different from knowing if a clinical trial result is statistically significant. NNT allows us to place a number on how often we can expect to encounter a difference between two interventions. If we see a therapeutic difference once every 100 patients (NNT of 100), the difference between the treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 7 patients being treated with an intervention vs another (NNT of 7), the result will likely influence dayto-day practice.
How to calculate NNT (or NNH):
What is the NNT for an outcome for drug A vs drug B?
fA = frequency of outcome for drug A
fB = frequency of outcome for drug B
NNT = 1/[ fA - fB]
By convention, we round up the NNT to the next higher whole number.
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to encounter a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates: .75 -.55 = .20
- NNT: 1/.20 = 5
A rule of thumb: NNT values for a medication vs placebo <10 usually denote a medication we use on a regular basis to treat patients.
a Adapted from Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71. Citrome L. Can you interpret confidence intervals? It’s not that difficult. Current Psychiatry. 2007;6(8):77-82. Additional information can be found in Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411 (free to access at onlinelibrary.wiley.com/doi/full/10.1111/ijcp.12142)
Overall tolerability and safety
The systemic safety and tolerability profile for asenapine transdermal system would be expected to be similar to that for sublingual asenapine, unless there are adverse events that are related to high peak plasma concentrations or large differences between peak and trough plasma concentrations.6 Nonsystemic local application site adverse events would, of course, differ between sublingual vs transdermal administration.
Continue to: Use of asenapine transdermal system...