#Targets and #neuron
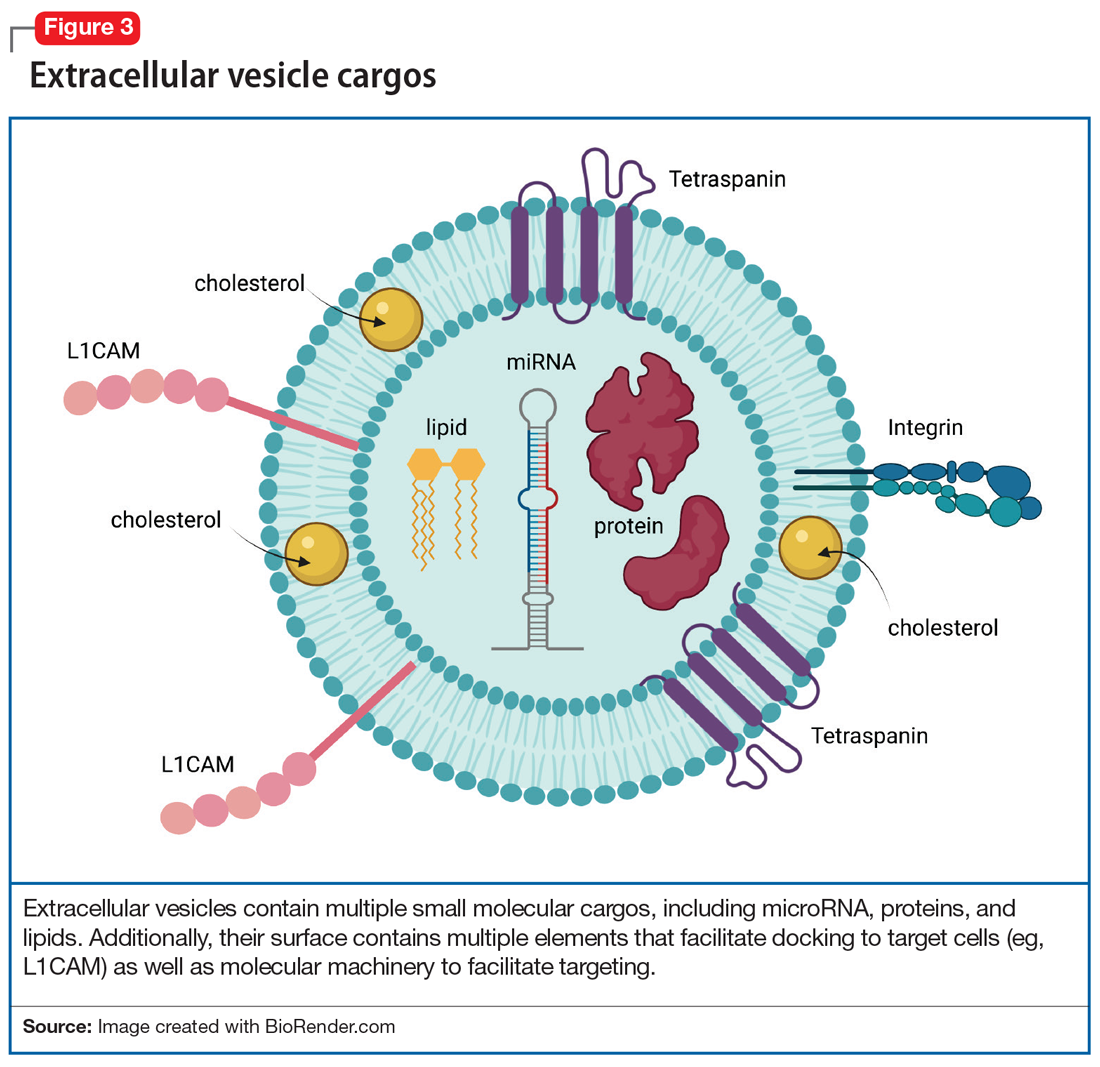
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.
EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/