Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
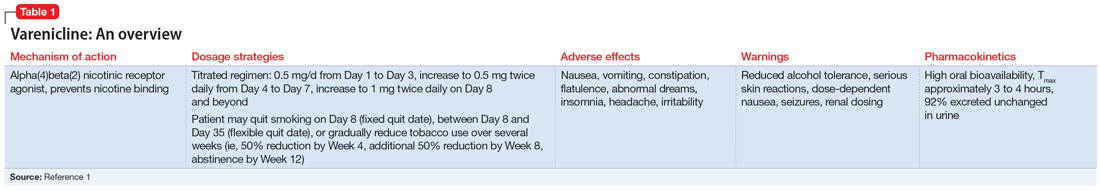
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
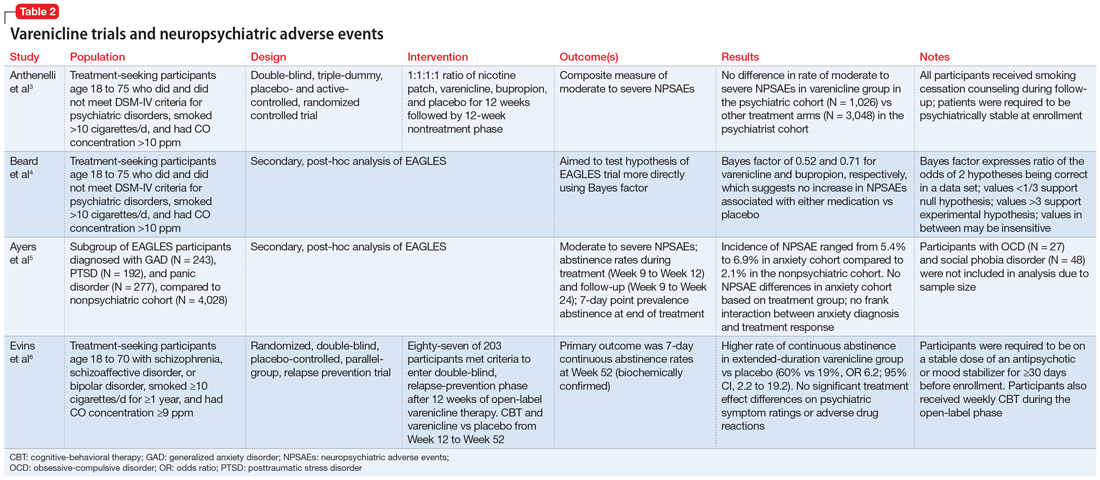
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES