The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
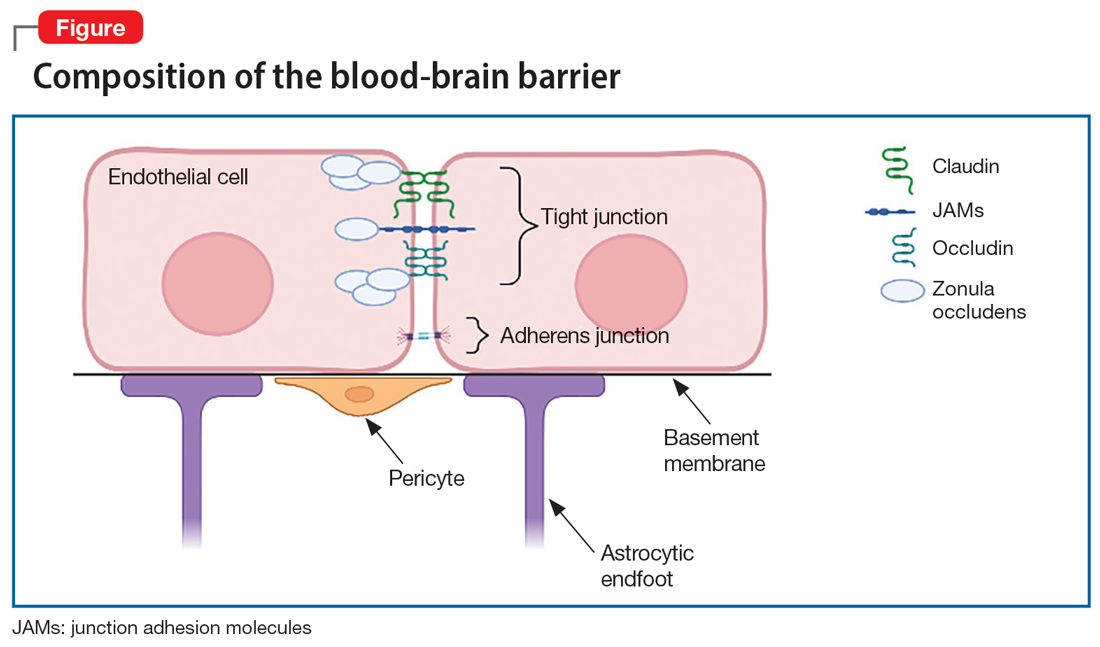
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...