Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
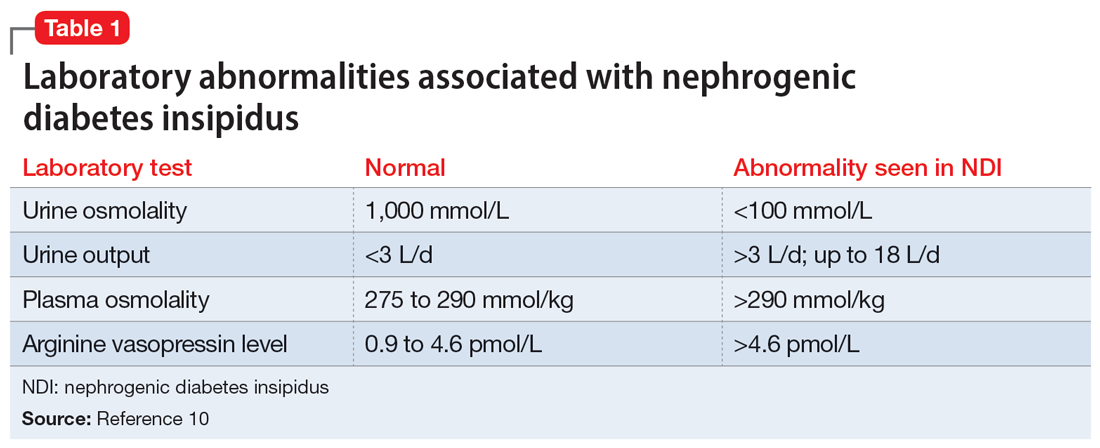
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10
Several treatment options
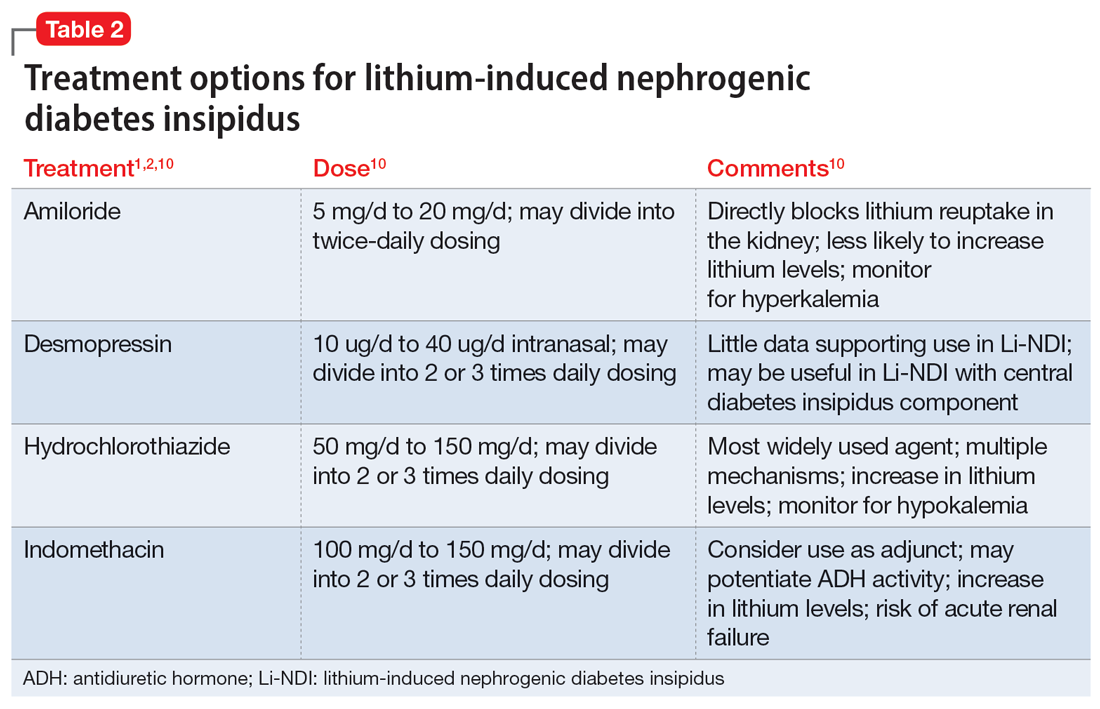
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2 Table 21,2,10 summarizes potential treatment options.
Continue to: Amiloride has the most...