Antipsychotics have long been linked with hyperprolactinemia.1 This phenomenon was first considered a drug class effect, but the arrival of clozapine, better deliniation of dopamine receptor subtypes, and identification of the four principal CNS dopamine pathways revealed that hyperprolactinemia was not a universal consequence of antipsychotic use.
We now know that most atypical antipsychotics are less likely to induce hyperprolactinemia than older antipsychotics, but we don’t know why. The most likely explanation is that most of the newer agents block dopamine D2 minimally in the hypothalamic tuberoinfundibular pathway.2 Evidence is emerging that atypical agents elevate serum prolactin levels at least transiently—but usually less than typical antipsychotics—and this effect varies, depending on each compound’s dopamine-binding properties.
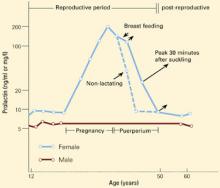
Figure 1 CHANGES IN PROLACTIN LEVELS OVER TIME
Mean serum prolactin concentrations from puberty until menopause. For nursing women, the length of the arrows depicts the increase in serum prolactin concentration associated with each episode of suckling. The Y-axis expresses serum prolactin concentration in both ng/ml and mg/l.
Source: Adapted and reprinted with permission from Friesen HG. Human prolactin. Ann R Coll Phys Surg Can 1978;11:275-81.
Prolactin physiology
Prolactin—a large peptide containing 198 amino acids—was the first anterior pituitary gland hormone to be isolated in pure form.3 Despite its molecular weight of approximately 23,000, the hormone easily crosses the blood-brain barrier.4
Similar to other anterior pituitary hormones, prolactin is secreted episodically. Its secretion is inhibited by dopamine release from the hypothalamus and enhanced by different prolactin-releasing factors. Prolactin is the only anterior pituitary hormone that is produced by tuberoinfundibular neurons governed by dopamine.5 Dopamine stimulates lactotrope D2 receptors and inhibits adenylate cyclase, resulting in reduced prolactin synthesis and release.
Serum prolactin concentrations change during various life stages (Figure 1).6 Estrogen’s effects on prolactin gene expression regulate prolactin synthesis, resulting in higher prolactin levels in premenopausal women than in men.
Prolactin secretion
Normally, prolactin is secreted in pulses—approximately 14 in a 24-hour period, with an interpulse interval of about 80 minutes.5 A bimodal daily pattern of secretion is superimposed upon this pattern, with peak levels at night and trough levels at noon. Stress—including surgery and general anesthesia, exercise, and hypoglycemia—may transiently increase prolactin levels.
Endocrine regulation. Estrogen modulates the response of hypothalamic factors that control prolactin production. It stimulates decreased prolactin response to dopamine and increased response to thyrotropic-releasing hormone.
Insulin also stimulates prolactin secretion—probably by inducing hypoglycemia. Serum insulin level changes within physiologic ranges appear to affect prolactin regulation.
Neuroendocrine regulation. The hypothalamus blunts prolactin secretion primarily via dopamine release. This modulation occurs principally within the tuberoinfundibular dopamine pathway. The D2 subtype is the only dopamine receptor in the anterior pituitary gland:
- a decrease in dopamine levels reaching the anterior pituitary gland increases the number of D2 receptors
- to a lesser extent, estrogen decreases the number of D2 receptors.
Dopamine-modulated reductions in action potential discharge from lactotrophs and in calcium flux leads to decreased intracellular calcium and decreased prolactin secretion.5
Most hormones are target-organ agents and are regulated via a feedback loop that includes the peripheral circulation. Prolactin, however, is not considered to have a specific target organ. It is its own inhibiting factor, using an autoregulatory, pituitary-to-hypothalamus short-loop feedback circuit.
For example, prolactin-secreting tumors or drugs that elevate hormone levels lead to an increase in dopamine. In contrast, hypophysectomy decreases dopamine. In this setting, prolactin injections will restore normal dopamine levels. Prolactin-releasing factors include thyrotropic-releasing hormone, vasoactive intestinal peptide, and serotonin.
Prolactin’s actions
Many tissues—including breast, liver, ovary, testis, and prostate—have prolactin receptors. These receptors are stimulated with equal potency by prolactin and growth hormone.
The principal site of prolactin action is the mammary gland, where the hormone initiates and maintains lactation after childbirth. Major stimuli for breast development are estrogen, progesterone, prolactin, and placental mammotropic hormones. Other stimuli include insulin, cortisol, and thyroid hormone.7
Gonadotropin secretion is influenced by prolactin via the hypothalamus. Prolactin-mediated inhibition of luteinizing hormone-releasing hormone secretion impairs gonadotropin release and inhibits gonadal function.
Table 1
COMMON CLINICAL EFFECTS IN PATIENTS WITH HYPERPROLACTINEMIA
| Organ or syndrome | Clinical effects |
|---|---|
| Behavior | Direct effects Secondary effects due to hypogonadism Possible cognitive impairment |
| Bones | Decreased bone mineral density due to testosterone or estrogen deficits |
| Breast | Engorgement Lactation unrelated to breast feeding |
| Cardiovascular system | Possible adverse effects due to low levels of testosterone or estrogen |
| Menstrual function | Absence of ovulation Amenorrhea |
| Sexual function | Reduced libido Reduced arousal Orgasmic dysfunction |
| Source: Adapted and reprinted with permission from Dickson RA, Glazer WM. Neurolepticinduced hyperprolactinemia. Schizophr Res 1999;35(suppl):S75-S86. | |
Diagnosis of hyperprolactinemia
Pathologic hyperprolactinemia is defined as consistently elevated serum prolactin concentration (>20 ng/ml) in the absence of pregnancy or postpartum lactation. Because of the pulsatile nature of prolactin secretion, a definitive diagnosis of hyperprolactinemia requires three serum prolactin levels taken on different mornings.