Biomarkers can help psychiatrists monitor abstinence and detect relapse in patients with alcohol use disorders. A blood test that measures carbohydrate-deficient transferrin percentage (%CDT) is FDA-approved for detecting heavy drinking (Table 1)1 and has shown effectiveness in several medical and surgical uses.
Table 1
%CDT test: Fast facts
| Brand names: |
| Axis-Shield %CDT, Bio-Rad %CDT TIA, Tina-quant (a) %CDT |
| FDA-approved indication: |
| Testing for excessive alcohol use |
| Manufacturers: |
| Axis-Shield PLC, Bio-Rad Laboratories, Roche Diagnostics |
| Recommended use: |
| Detecting heavy alcohol consumption, monitoring abstinence, and identifying elapse in patients with alcohol use disorders |
| Laboratories that process %CDT results: |
|
HOW %CDT SIGNALS ALCOHOL ABUSE
In 1976, Swedish researchers detected transferrin fractions in the CSF of alcoholic patients but not in nonalcoholics.2 One of these fractions was also present in alcoholics’ serum. This discovery led to the CDT biomarker.
%CDT testing is based on the finding that consuming an average >60 grams of alcohol (about 5 standard drinks) daily for ≥ 2 weeks causes a higher percentage of transferrin—a glycoprotein that transports iron in the blood—to lack its usual carbohydrate content. Transferrin appears in 6 isoforms, and studies have shown that heavy drinking increases three of these—asialo, mono sialo, and disialo transferrin—a state collectively called “carbohydrate-deficient.”
A %CDT reading ≥ 2.6 indicates that a patient may have had on average at least 5 alcoholic drinks daily for ≥ 2 weeks. Because CDT has a short mean half-life (7 to 14 days), readings >2.6 may suggest much heavier drinking at some time before the blood sample was taken.
Microcolumn chromatography separation assays that measure CDT as a percentage of total circulating transferrin have replaced initial CDT assays that used isoelectric focusing separation and immunoassay systems. This advance corrected for individual transferrin level variations.
CLINICAL USE
The %CDT test has shown effectiveness in several medical and surgical uses, including
- screening patients with diseases possibly triggered by alcohol use, such as treatment-resistant hypertension, gastroesophageal reflux disease, or depression
- detecting alcohol-use disorders in hospitalized patients
- screening presurgical and trauma patients to predict alcohol withdrawal syndrome and/or postsurgical complications.3,4
Although no alcohol biomarker alone reliably confirms alcohol abuse/dependence, %CDT can corroborate initial clinical impressions, especially when the patient’s self-report is suspect or information from significant others is not available.
Among biomarkers, %CDT most accurately predicts alcohol withdrawal syndrome in men (mean corpuscular volume [MCV] measurements are more accurate in women).4 During psychiatric consults, the test may help confirm suspected alcohol use in patients admitted to inpatient medical, surgical, or trauma units.
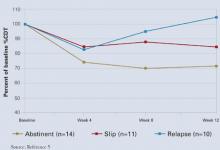
%CDT may also help monitor abstinence when treating an alcohol use disorder. In a major prospective treatment outcome study, %CDT was more sensitive than gamma-glutamyltransferase (GGT) in detecting relapse in male but not female alcoholics.5 Patients who abstained from drinking for 12 weeks showed a 30% decrease in %CDT. Subjects who relapsed during treatment later showed a 60% increase in %CDT, indicating sustained heavy drinking.
%CDT decreased over the first 4 weeks of treatment and continued to decline over time for abstinent patients (Figure). After week 4, %CDT increased again for those who consumed ≥ 5 drinks per day or had a full-blown relapse.5
Together, routine %CDT and GGT testing during treatment and follow-up can help clinicians monitor a patient’s progress and provide accurate reports to courts, child welfare agencies, and programs for impaired professionals. A 30% reduction from baseline in either biomarker indicates abstinence or significantly reduced alcohol consumption, whereas a 30% increase suggests relapse.6 Consider all clinical information in the final analysis, however.
%CDT results also provide an objective basis for discussing relapses with patients. Those who are responding to treatment often welcome %CDT testing as proof they are abstaining from alcohol.
Figure %CDT changes with alcohol use or abstinence
CLINICAL PRACTICALITY
The %CDT test is an immunoassay with ion-exchange column separation followed by turbidimetric measurement. Because the procedure is complex, laboratories generally require 24 to 72 hours to test the sample.
Because the %CDT test is relatively new, pricing, reimbursement rates, and availability are not well established. Pricing will likely vary widely from state to state and among laboratories and insurers. Medicare reimburses approximately $25 for %CDT testing, compared with $10 for GGT testing.
Only select reference laboratories offer %CDT testing, but the test should become more widely available over time (Table 1).
SENSITIVITY AND SPECIFICITY
%CDT test sensitivity is 70% to 90% in male inpatient alcoholics (usually drinking within 4 to 7 days of blood draw), with specificities >90%. Sensitivity is 12% to 40% in general populations—among whom underreporting of drinking may lead to more false positives—and 40% to 60% in outpatient alcoholics.7,8 Specificity, however, remains high—80% to 90%—when sensitivity is reduced.