A new, FDA-approved in-office lithium test (Table) can eliminate the inconvenience and fallibility of testing venous blood samples that often discourage lithium use. The test, which measures lithium in capillary blood drawn from a finger stick, has shown reliability when compared in clinical trials with established testing methods.
Why finger-stick testing?
Periodically monitoring serum or plasma lithium minimizes side effects and toxicity, maintains therapeutic dosing, and ensures treatment adherence. Laboratories generally use flame photometry, atomic absorption (AA) spectrophotometry, or ion-selective electrode analysis to measure lithium in blood drawn via venipuncture. A colorimetric assay is also available.1
Table
Lithium fingerstick test: Fast facts
| Brand name: |
| InstaRead Lithium System |
| Indication: |
| Testing plasma lithium levels in-office |
| Manufacturer: |
| ReliaLAB |
| Recommended use: |
| Testing plasma lithium levels 12 hours after dosing; repeat test after 5 minutes to confirm abnormal reading |
| Reimbursement information: |
| 1-866-467-8273 or www.relialab.com/Reimbursement.html |
For years, researchers have investigated alternatives to venipuncture lithium testing. Aside from being inconvenient, venipuncture draws can increase risk of excessive bleeding, hematoma, infection, vasovagal syncope, and multiple punctures to locate a vein. In some cases:
- psychiatrists wait 2 or more days for a laboratory to return results
- patients forget to have blood drawn before the office visit
- samples are incorrectly timed in relation to the last dose
- results are filed away unnoticed
- or the psychiatrist needs to call the patient 3 days or so after the visit to discuss an abnormal reading.
With the new in-office test, clinicians can ensure they will obtain a valid blood sample in minutes, 12 hours after dosing. Psychiatrists then can immediately discuss the result with patients, perform a repeat test 5 minutes later to check an abnormal reading, and counsel patients on raising low lithium levels. This instant feedback can powerfully reinforce a physician’s advice and promote treatment adherence.2
How it works
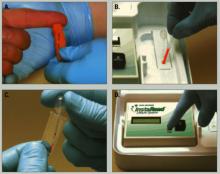
A 50-μl blood sample is drawn via finger stick and converted to plasma in a lectin-coated membrane separator. The clinician then adds 0.2 μl of the plasma to a micro-cuvette containing a colorimetric reagent that is photometrically analyzed for lithium. The test takes 5 minutes or less (Figure).
The assay has been shown to be sensitive to 0.1 mEq/L of lithium and linear between 0.1 and 2.5 mEq/L.3
Figure How finger-stick lithium test works
Clinician obtains blood sample (a) and empties it into a separator (b), which processes blood to plasma. Clinician then adds plasma to a reagent vial (c), which is inserted into a reader (d) to obtain a lithium level.
Reliability
In clinical trials during which patients were tested and retested, the colorimetric assay showed reliability when compared with:
- routine lithium spectrophotometry. Researchers compared venipuncture blood samples split for colorimetric and spectrophotometric testing
- atomic absorption spectrophotometry of venipuncture blood from psychiatric patients
- standard spectrophotometry of venipuncture samples to which a known amount of lithium was added.4
Colorimetric finger-stick testing also was compared with AA spectrophotometry testing of 88 matched venipuncture samples from 56 bipolar patients.5 Results were not identical, but most fingerstick results varied no more than±0.2 mEq/L from the AA results. Differences were positive and negative, indicating random variation between the two methods rather than systematic bias.
Clinical applicability
In-office finger-stick blood testing for lithium levels could improve quality of care for patients taking lithium.
The manufacturer, ReliaLAB, says the test costs $399, plus $264 for a refill kit containing 24 patient test packs. A certain volume of patients taking lithium would seem to be necessary to justify purchasing the instrument.
The test may be reimbursable under certain circumstances. ReliaLAB offers information on coding and reimbursement for in-office lithium monitoring (Table).
Also, because instant in-office creatinine and thyroid-stimulating hormone tests are not available, lithium therapy monitoring will still require laboratory visits when these tests are needed. Nonetheless, point-of-care plasma lithium level determination should improve convenience, compliance, and overall comprehensiveness of care.
Related resources
- Online information on in-office lithium test. www.relialab.com/Lith.html.
- Johnson FN. The origins of lithium therapy. Rev Contemp Pharmacother 1999;10:193-265.
Drug brand names
- Lithium • Eskalith, others
Disclosure
Dr. Jefferson reports no financial relationship with or proprietary interest in ReliaLAB.