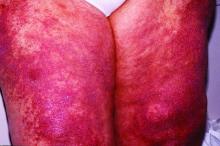
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.