Industry-sponsored research funding has fallen by more than 20% from 2014 to 2022, according to a new analysis.
“Despite the growing partnerships and networks between rheumatologists, the public sector, and the health care industry to optimize research funding allocations, the declining trend in industry-sponsored research payments is a concerning sign for all rheumatologists,” writes study author Anju Murayama, an undergraduate medical student at the Tohoku University School of Medicine in Sendai City, Japan. The data suggest that “more and more rheumatologists are facing difficulties in obtaining research funding from the health care industry.”
Dr. Murayama used the Open Payments Database, which contains records of payments made by drug and pharmaceutical companies to health care providers. The analysis included research payments provided directly to rheumatologists (direct-research payments) and payments given to clinicians or health care organizations related to research whose principal investigator was a rheumatologist (associated-research payments). These associated payments included costs for study enrollment and screening, safety monitoring committees, research publication, and more.
The research was published August 15 in The Journal of Rheumatology .
In 2014, the total direct payments to rheumatologists from industry were $1.4 million. These payments jumped to nearly $4.6 million in 2016 but have declined since. In 2022, there were $976,481 in total payments, a 31% drop from 9 years before.
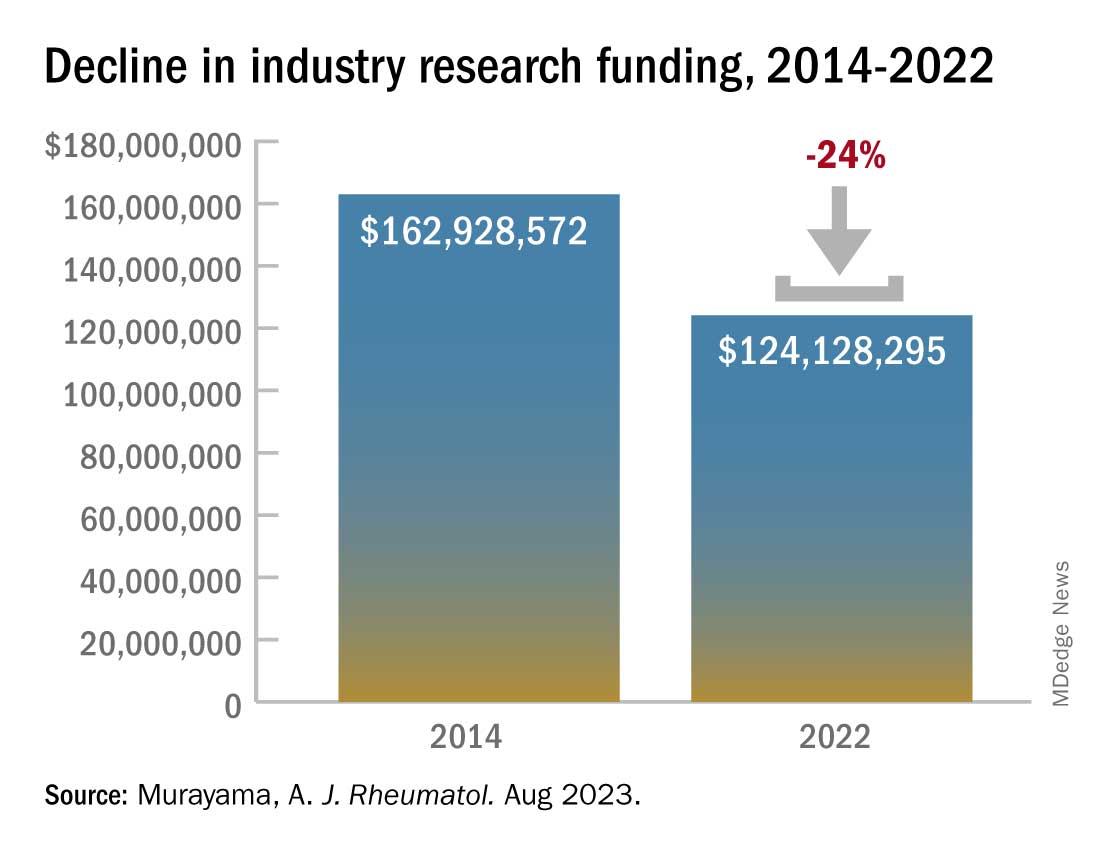
Associated payments dwarfed direct payments with a total of $162.9 million paid by the industry in 2014. These payments reached $217.7 million in 2015 but dropped over the next 7 years. In 2022, associated payments totaled $124.1 million, a 24% decrease from 2014.This decline comes after an observed drop in research funding from the public sector. From 2014 to 2017, public-sector research funding to members of the American College of Rheumatology fell by 7.5%. Timothy Niewold, MD, a rheumatologist and vice chair for research in the department of medicine at Hospital for Special Surgery, New York, said that he and colleagues have felt the funding squeeze from both public and industry sectors. “The budgets for trials have seemed tight,” he told this news organization. With the overhead and cost of doing a trial at an academic institution like HSS, “sometimes you can’t make the budget work,” and researchers must pass on industry-funded trials.
The analysis also found a larger discrepancy between average and median associated-research payments. Of the $1.4 billion in associated-research payments combined over the 9-year period, the median payments per physician ($173,022) were much smaller than the mean payments ($989,753), which indicates that “only a very small number of rheumatologists received substantial amounts of research funding from the industry,” Dr. Murayama wrote in an email to this news organization. “This finding might support statements published by Scher and Schett in Nature Review Rheumatology suggesting that many industry-initiated clinical trials are conducted and authored by a small number of influential rheumatologists, often referred to as key opinion leaders.”
The analysis also found that of all associated payments, less than 3% ($39.2 million) went to funding preclinical research, which is “more disappointing than surprising,” Dr. Niewold said. Though clinical trials are expensive and require larger amounts of investment, industry partnerships at preclinical phases of research are important for devising novel solutions for these complex rheumatic diseases, he noted. “The clinical trials are one piece,” he added, “but you need the whole [research] continuum.”
Dr. Niewold reports receiving research grants from EMD Serono and Zenas Biopharma and consulting for Thermo Fisher Scientific, Progentec Diagnostics, Roivant Sciences, Ventus, S3 Connected Health, AstraZeneca, and Inova. Dr. Murayama reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.