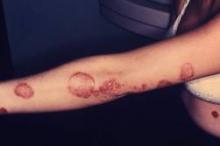
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.