User login
Looks Aren’t Everything in Breast Reconstruction
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Pudendal Neuralgia
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
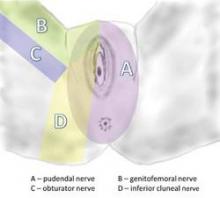
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
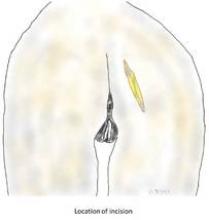
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
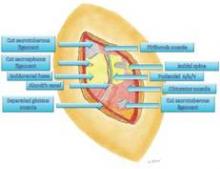
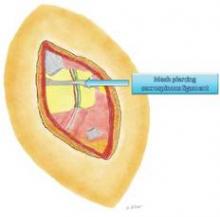
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
The Hoopla Over Mesh: What It Means for Practice
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
The Where and Why of Postsurgical Adhesions
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
Endometriosis: Current Diagnosis and Treatment
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
The Benefits of Robot-Assisted Myomectomy
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
The Ever-Changing Laparoscopic Myomectomy
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.