User login
Bowel perforation causes woman’s death: $1.5M verdict
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A 46-year-old woman underwent laparoscopic supracervical hysterectomy to remove her uterus but preserve her cervix. Postsurgically, she had difficulty breathing deeply and reported abdominal pain. The nurses and on-call physician reassured her that she was experiencing “gas pains” due to insufflation. After same-day discharge, she stayed in a motel room to avoid a second-floor bedroom at home.
She called the gynecologist’s office the following day to report continued pain and severe hot flashes and sweats. The gynecologist instructed his nurse to advise the patient to stop taking her birth control pill (ethinyl estradiol/norethindrone, Microgestin) and “to ride out” the hot flashes.
The woman was found dead in her motel room the next morning. An autopsy revealed a perforated small intestine with leakage into the abdominal cavity causing sepsis, multi-organ failure, and death.
ESTATE’S CLAIM The gynecologist reviewed the medical records and found an error in the operative report, but he made no addendum or late entry to correct the operative report. His defense counsel instructed him to draft a letter clarifying the surgery; this clarification was given to defense experts. The description of the procedure in the clarification was different from what was described in the medical records. For example, the clarification reported making 4 incisions for 4 trocars; the operative report indicated using 3 trocars. The pathologist and 2 nurses who treated the patient after surgery confirmed that there were 3 trocar incisions. The pathologist found no tissue necrosis at or around the perforation site, indicating that the perforation likely occurred during surgery.
PHYSICIAN’S DEFENSE Bowel perforation is a known complication of the procedure. The perforation was not present at the time of surgery because leakage of bowel content would have been obvious.
VERDICT A $1.5 million Virginia settlement was reached.
Retained products of conception after D&C
When sonography indicated that a 30-year-old woman was pregnant, she decided to abort the pregnancy and was given mifepristone.
Another sonogram 5 weeks later showed retained products of conception within the uterus. An ObGyn performed dilation and curettage (D&C) at an outpatient clinic. Because he believed the cannula did not remove everything, he used a curette to scrape the uterus. After the patient was dizzy, hypotensive, and in pain for 4 hours, an ambulance transported her to a hospital. Perforations of the uterus and sigmoid colon were discovered and repaired during emergency surgery. The patient has a large scar on her abdomen.
PATIENT'S CLAIM The ObGyn did not perform the D&C properly and perforated the uterus and colon. An earlier response to symptoms could have prevented repair surgery. Damage to the uterus may now preclude her from having a successful pregnancy.
DEFENDANTS’ DEFENSE The ObGyn argued that the aborted pregnancy was ectopic; spontaneous rupture caused the perforations.
VERDICT A $340,000 New York settlement was reached with the ObGyn. By the time of trial, the clinic had closed.
Wrong-site biopsy; records altered
A 40-year-old woman underwent excisional breast biopsy. The wrong lump was removed and the woman had to have another procedure.
PATIENT'S CLAIM The hospital’s nursing staff failed to properly mark the operative site. The breast surgeon did not confirm that the markings were correct. The surgeon altered the written operative report after the surgery to conceal negligence.
DEFENDANTS’ DEFENSE The nurses properly marked the biopsy site, but the surgeon chose another route. The surgeon edited the original report to reflect events that occurred during surgery that had not been included in the original dictation. The added material gave justification for performing the procedure at a different site than originally intended.
VERDICT A $15,500 Connecticut verdict was returned.
Second twin has CP and brain damage: $10M settlement
A woman gave birth to twins at an Army hospital. The first twin was delivered without complications. The second twin developed a prolapsed cord during delivery of the first twin. A resident and the attending physician allowed the mother to continue with vaginal delivery. The heart-rate monitor showed fetal distress, but the medical staff did not respond. After an hour, another physician was consulted, and he ordered immediate delivery. The attending physician decided to continue with vaginal delivery using forceps, but it took 15 minutes to locate forceps in the hospital. The infant suffered severe brain damage and cerebral palsy. She will require 24-hour nursing care for life, including treatment of a tracheostomy.
PARENTS' CLAIM The physicians were negligent for not reacting to non-reassuring monitor strips and for allowing the vaginal delivery to continue. An emergency cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $10 million North Carolina settlement was reached for past medical bills and future care.
Faulty biopsies: breast cancer diagnosis missed
In September 2006, a 40-year-old woman underwent breast sonography. A radiologist, Dr. A, reported finding a mass and a smaller nodule in the right breast, and recommended a biopsy of each area. Two weeks later, a second radiologist, Dr. B, biopsied the larger of the two areas and diagnosed a hyalinized fibroadenoma. He did not biopsy the smaller growth, but reported it as a benign nodule. He recommended more frequent screenings. The patient was referred to a surgeon, who determined that she should be seen in 6 months.
In June 2007, the patient underwent right-breast sonography that revealed cysts and three nodules. The surgeon recommended a biopsy, but the biopsy was performed on only two of three nodules. A third radiologist, Dr. C, determined that the nodules were all benign.
In November 2007, when the patient reported a painful lump in her right breast, her gynecologist ordered mammography, which revealed lesions. A biopsy revealed that one lesion was stage III invasive ductal carcinoma. The patient underwent extensive treatment, including a mastectomy, lymphadenectomy, chemotherapy, and radiation therapy, and prophylactic surgical reduction of the left breast.
PATIENT'S CLAIM The cancer should have been diagnosed in September 2006. Prompt treatment would have decreased the progression of the disease. The September 2006 biopsy should have included both lumps, as recommended by Dr. A.
DEFENDANTS’ DEFENSE There was no indication of cancer in September 2006. Reasonable follow-up care was given.
VERDICT A New York defense verdict was returned.
Tumor not found during surgery; BSO performed
A 41-year-old woman underwent surgery to remove a pelvic tumor in November 2004. The gynecologist was unable to locate the tumor during surgery. He performed bilateral salpingo-oophorectomy (BSO) because of a visual diagnosis of endometriosis. In August 2005, the patient underwent surgical removal of the tumor by another surgeon. She was hospitalized for several weeks and suffered a large scar that required additional surgery.
PATIENT'S CLAIM BSO was unnecessary, and caused early menopause, with vaginal atrophy and dryness, depression, fatigue, insomnia, loss of hair, and other symptoms.
The patient claimed lack of informed consent. From Ecuador, the patient’s command of English was not sufficient for her to completely understand the consent form; an interpreter should have been provided.
DEFENDANTS’ DEFENSE BSO did not cause a significant acceleration of the onset of menopause. It was necessary to treat the endometriosis.
The patient signed a consent form that included BSO. The patient did not indicate that she did not understand the language on the form; had she asked, an interpreter would have been provided.
VERDICT A $750,000 New York settlement was reached with the gynecologist and medical center.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Urinary incontinence affects more than one-third of women aged 70 years or older. As the authors of this study point out, urinary incontinence is comparable to other chronic conditions, such as hypertension and diabetes mellitus, in its impact on quality of life, and is a common reason for institutionalization.
The risk of urinary incontinence increases with age. In elderly women, it is often mixed (ie, having both urge- and stress-related components) and associated with functional impairments, including reduced mobility. It is thought that the association between incontinence and functional impairment is related primarily to the urge component:
- Women with impaired mobility take longer to reach the toilet, increasing the risk of leakage when the urge to urinate is strong
- Women with urge incontinence may be more likely to limit their activities so that they are always near a toilet.
Cognitive impairment may also play a role, affecting motor skills and bladder control.
To better understand why advancing age is linked with urinary incontinence, Fritel and colleagues studied a population of 1,942 urban-dwelling French women aged 75 to 85 years (mean age, 79.3 years; mean body mass index, 25.9 kg/m2).
Details of the study
Investigators assessed the frequency and quantity of urine leaks, the impact of urinary incontinence on daily life, and the participants’ mobility and balance. Data on urinary incontinence were collected via a self-administered questionnaire (the International Consultation on Incontinence Questionnaire–Short Form). Motor-related physical function was assessed using standardized balance and gait tests.
Urinary incontinence was reported by 42% of participants. Of these women, 57% reported daily urine leakage, with mixed incontinence found to be more prevalent than urge incontinence, which was more prevalent than stress incontinence. Overall, women with urinary incontinence reported that its impact on daily life was mild. Among those with mixed or urge incontinence, limitations in mobility and balance were correlated significantly with the severity of incontinence.
What this evidence means for practice
These findings support earlier data suggesting that impaired mobility can promote urge and mixed urinary incontinence. Because there also is a possibility that cerebral deterioration in aging women causes gait and balance problems that increase the likelihood of urge incontinence, the authors advise against the use of anticholinergic agents in elderly women, as these drugs can impair cognitive function. Another take-home message from this French report is that we should counsel elderly patients about the importance of maintaining balance and mobility through exercise, physical therapy, or other strategies.
Andrew M. Kaunitz, MD
Imperforate hymen in your adolescent patient: Don’t miss the diagnosis
Many gynecologists encounter imperforate hymen, a congenital vaginal anomaly, in general practice. As such, it is important to have a basic understanding of the condition and to be aware of appropriate screening, evaluation, and management. This knowledge will allow you to differentiate imperforate hymen from more complex anomalies—preventing significant morbidity that could result from performing the wrong surgical procedure on this condition—and to provide optimal surgical management.
How often and why does it occur?
Imperforate hymen occurs in approximately 1/1000 newborn girls. It is the most common obstructive anomaly of the female reproductive tract.1,2
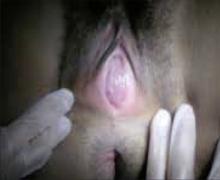
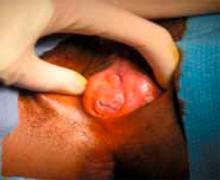
The hymen consists of fibrous connective tissue attached to the vaginal wall. In the perinatal period, the hymen serves to separate the vaginal lumen from the urogenital sinus (UGS); this is usually perforated during embryonic life by canalization of the most caudal portion of the vaginal plate at the UGS. This establishes a connection between the lumen of the vaginal canal and the vaginal vestibule.3 Failure of the hymen to perforate completely in the perinatal period can result in varying anomalies, including imperforate (FIGURE 1), microperforate, cribiform, or septated hymen.
Figure 1. Imperforate hymen
How does it present?
Its presentation is variable and frequently asymptomatic in infants and children.4 As a result, the diagnosis is often delayed until puberty.3
In infancy. Newborns typically will present with a hymenal bulge from hydrocolpos or mucocolpos, which result from maternal estrogen secretion on the newborn’s vaginal epithelium.5 This is usually asymptomatic and self limited.
Rarely, large hydrocolpos/mucocolpos may become symptomatic and can lead to urinary obstruction, or they can present as an abdominal mass or intestinal obstruction.4
In adolescence. The majority of adolescents will present with cyclic or persistent pelvic pain and primary amenorrhea. If significant hematometra is present, an abdominal mass also may be palpated. In extreme cases, the patient may present with mass effect symptoms, including back pain, pain with defecation, constipation, nausea and vomiting, urinary retention, or hydronephrosis.6 Retrograde passage of blood into the fallopian tubes can cause hematosalpinx, which can lead to endometriosis and adhesion formation. Blood also may pass freely into the peritoneal cavity, forming hemoperitoneum.3
Related article: Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Imperforate hymen, vaginal septum, or distal vaginal atresia?
When in doubt, refer. Imperforate hymen can be confused with distal vaginal atresia or low transverse vaginal septum. Often, the patient may present with similar signs and symptoms in all 3 cases. Accurately differentiating imperforate hymen from the former two more complex congenital anomalies prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity. As such, it is important to appropriately define the anatomy and refer the complex cases to a specialist comfortable and skilled in managing congenital anomalies, usually a pediatric and adolescent gynecologist or reproductive endocrinologist.
Imperforate hymen
Examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.7 This finding, coupled with a rectal examination and pelvic ultrasonography is usually sufficient to make the diagnosis.6,8 However, magnetic resonance imaging (MRI) of the pelvis should be obtained in cases where the diagnosis is uncertain or the physical exam is more consistent with vaginal septum or agenesis.
Transverse vaginal septum
A reverse septum results from failure of the müllerian duct derivatives and UGS to fuse or canalize. This can occur in the lower, middle, or upper portion of the vagina, and septa may be thick or thin.6 Low transverse septa are more easily confused with imperforate hymen. Examination usually reveals a normal hymen with a short vagina posteriorly. In cases of extreme hematocolpos, vaginal septa also may present with a perineal bulge but, again, this will be posterior to a normal hymen.
Distal vaginal atresia
This condition occurs during embryonic development when the UGS fails to contribute to the lower portion of the vagina (FIGURE 2).5 In cases of distal vaginal atresia there is a lack of vaginal orifice, or only a vaginal dimple may be present.5,6 Rectovaginal examination will reveal a palpable mass if the upper vagina is distended with blood.6
Figure 2. Lower vaginal atresia
MRI is vital to firm diagnosis
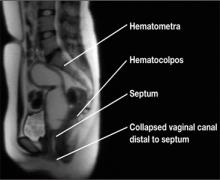
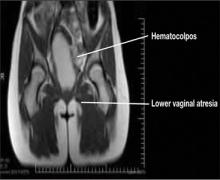
In addition to pelvic ultrasonography, pelvic MRI is necessary to delineate the anatomy with both vaginal septum (FIGURE 3) and lower vaginal atresia (FIGURE 4), as preoperative evaluation of location and thickness of a vaginal septum as well as measurement of the total length of agenesis is imperative.6-8 Misdiagnosis of the vaginal septa or atresia as an imperforate hymen can lead to significant scarring and stenosis and can make corrective surgical procedures difficult or suboptimal.
| Figure 3. MRI of transverse vaginal septum |
Figure 4. MRI of lower vaginal atresia
Surgical management: hymenectomy
Imperforate hymen is managed surgically with hymenectomy. Repair is generally reserved for the newborn period or, ideally, in adolescence, as at puberty the presence of estrogen aids in surgical repair and healing.5 Simple aspiration of hematocolpos/ mucocolpos can lead to ascending infection, and pyocolpos and should be avoided.6
The goal of hymenectomy is to:
-
open the hymeneal membrane to allow egress of fluid and menstrual flow
-
allow for tampon use
The procedure is relatively straightforward and usually is performed under general anesthesia, although regional anesthesia also is an option.
Steps to the varying hymenectomy incisions
Cruciate incision
1. Incise the hymen at the 2-, 4-, 8-, and 10-o’clock positions into four quadrants.
2. Excise the quadrants along the lateral wall of the vagina.
Elliptical incision
1. Make a circumferential incision with the Bovie electrocautery, incising the hymenal membrane close to the hymenal ring.
U-incision
1. Similar to the elliptical incision, use the Bovie electrocautery to incise the tissue close to the hymenal ring posterior and laterally in a “u” shape.
2. Make a horizontal incision superiorly to remove the extra tissue.
Vertical incision
This incision has been described in cases where there is an attempt to spare the hymen for religious or cultural preference.
1. Make a midline vertical hymenotomy less than 1 cm. Drain the borders of the hymen.
2. Apply suture obliquely to form a circular opening.
References
1. Dominguez C, Rock J, Horowitz I. Surgical conditions of the vagina and urethra. In: TeLinde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1997.
2. Basaran M, Usal D, Aydemir C. Hymen sparing surgery for imperforate hymen: case reports and review of literature. J Pediatr Adolesc Gynecol. 2009;22(4):e61–e64.
Tips to a successful procedure
Ensure adequate suctioning. Before starting the procedure, insert a Foley catheter to completely drain the bladder and delineate the urethra. Making an initial incision into the hymen usually results in the expulsion of the old blood and mucus, which can be very thick and viscous; therefore, it is important to have adequate suction tubing.
Prevent scarring. After evacuating the old blood and mucus, excise the hymeneal membrane with a cruciate incision as is traditionally described. Alternatively, some experts use an elliptical incision or u-incision. (See “Steps to the varying hymenectomy incisions”.) Prevent excision of the hymenal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis and dyspareunia.3
Suturing the mucosal margins is likely unnecessary in adolescent patients. After excision of the hymenal tissue, one option is to suture the mucosal margins of the hymenal ring in an interrupted fashion with a fine, delayed-absorbable suture. Alternatively, at our institution, where we employ the u-incision (FIGURE 5), we assure hemostasis of the mucosal margins and do not suture the margins. Suturing the margins is believed to prevent adherence of the edges; however, in the pubertal girl, adherence is unlikely secondary to estrogen exposure.
Figure 5. Surgical correction with u-incision
Avoid infection; do not irrigate. We do not recommend that you irrigate the vagina and perform unnecessary uterine manipulation, as this may introduce bacteria into the dilated cervix and uterus.3,8
Septate/microperforate/cribiform hymen
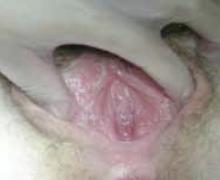
These other hymeneal anomalies also may require surgical correction if they become clinically significant. Patients may present with difficulty inserting or removing a tampon, insertional dyspareunia, or incomplete drainage of menstrual blood.6
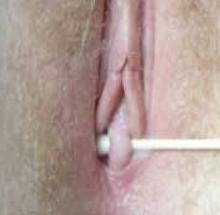
Imaging is usually not indicated to diagnose these hymenal anomalies, as physical examination will reveal a patent vaginal tract. A moistened Q-tip can be placed into the orifice or behind the septate hymen for confirmation (FIGURE 6).
Surgical correction of a microperforate or cribiform hymen is performed using the same principles as imperforate hymen.
Surgical correction of a septate hymen involves tying and suturing or clamping with a hemostat the upper and lower edges, with the excess hymenal tissue between the sutures then excised.8
Figure 6. Septate hymen
Postop care and follow up
Postoperative analgesia with lidocaine jelly or ice packs is usually sufficient for pain management. Reinforce proper hygienic care measures. At 2- to 3-week follow up, assess the patient for healing and evaluate the size of the hymenal orifice.
Key takeaways
-Differentiating imperforate hymen from low transverse vaginal septum or distal vaginal agenesis prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity.
-With imperforate hymen, examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.
-Pelvic MRI is essential to delineate the anatomy with both vaginal septum and agenesis, for preoperative evaluation of location and thickness of septum as well as measurement of total length of agenesis.
-Hymenectomy is relatively straightforward and may be performed using a cruciate, elliptical, or u-incision.
-Care should be taken to prevent excision of hymeneal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis, and later lead to dyspareunia.
Many gynecologists encounter imperforate hymen, a congenital vaginal anomaly, in general practice. As such, it is important to have a basic understanding of the condition and to be aware of appropriate screening, evaluation, and management. This knowledge will allow you to differentiate imperforate hymen from more complex anomalies—preventing significant morbidity that could result from performing the wrong surgical procedure on this condition—and to provide optimal surgical management.
How often and why does it occur?
Imperforate hymen occurs in approximately 1/1000 newborn girls. It is the most common obstructive anomaly of the female reproductive tract.1,2
The hymen consists of fibrous connective tissue attached to the vaginal wall. In the perinatal period, the hymen serves to separate the vaginal lumen from the urogenital sinus (UGS); this is usually perforated during embryonic life by canalization of the most caudal portion of the vaginal plate at the UGS. This establishes a connection between the lumen of the vaginal canal and the vaginal vestibule.3 Failure of the hymen to perforate completely in the perinatal period can result in varying anomalies, including imperforate (FIGURE 1), microperforate, cribiform, or septated hymen.
Figure 1. Imperforate hymen
How does it present?
Its presentation is variable and frequently asymptomatic in infants and children.4 As a result, the diagnosis is often delayed until puberty.3
In infancy. Newborns typically will present with a hymenal bulge from hydrocolpos or mucocolpos, which result from maternal estrogen secretion on the newborn’s vaginal epithelium.5 This is usually asymptomatic and self limited.
Rarely, large hydrocolpos/mucocolpos may become symptomatic and can lead to urinary obstruction, or they can present as an abdominal mass or intestinal obstruction.4
In adolescence. The majority of adolescents will present with cyclic or persistent pelvic pain and primary amenorrhea. If significant hematometra is present, an abdominal mass also may be palpated. In extreme cases, the patient may present with mass effect symptoms, including back pain, pain with defecation, constipation, nausea and vomiting, urinary retention, or hydronephrosis.6 Retrograde passage of blood into the fallopian tubes can cause hematosalpinx, which can lead to endometriosis and adhesion formation. Blood also may pass freely into the peritoneal cavity, forming hemoperitoneum.3
Related article: Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Imperforate hymen, vaginal septum, or distal vaginal atresia?
When in doubt, refer. Imperforate hymen can be confused with distal vaginal atresia or low transverse vaginal septum. Often, the patient may present with similar signs and symptoms in all 3 cases. Accurately differentiating imperforate hymen from the former two more complex congenital anomalies prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity. As such, it is important to appropriately define the anatomy and refer the complex cases to a specialist comfortable and skilled in managing congenital anomalies, usually a pediatric and adolescent gynecologist or reproductive endocrinologist.
Imperforate hymen
Examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.7 This finding, coupled with a rectal examination and pelvic ultrasonography is usually sufficient to make the diagnosis.6,8 However, magnetic resonance imaging (MRI) of the pelvis should be obtained in cases where the diagnosis is uncertain or the physical exam is more consistent with vaginal septum or agenesis.
Transverse vaginal septum
A reverse septum results from failure of the müllerian duct derivatives and UGS to fuse or canalize. This can occur in the lower, middle, or upper portion of the vagina, and septa may be thick or thin.6 Low transverse septa are more easily confused with imperforate hymen. Examination usually reveals a normal hymen with a short vagina posteriorly. In cases of extreme hematocolpos, vaginal septa also may present with a perineal bulge but, again, this will be posterior to a normal hymen.
Distal vaginal atresia
This condition occurs during embryonic development when the UGS fails to contribute to the lower portion of the vagina (FIGURE 2).5 In cases of distal vaginal atresia there is a lack of vaginal orifice, or only a vaginal dimple may be present.5,6 Rectovaginal examination will reveal a palpable mass if the upper vagina is distended with blood.6
Figure 2. Lower vaginal atresia
MRI is vital to firm diagnosis
In addition to pelvic ultrasonography, pelvic MRI is necessary to delineate the anatomy with both vaginal septum (FIGURE 3) and lower vaginal atresia (FIGURE 4), as preoperative evaluation of location and thickness of a vaginal septum as well as measurement of the total length of agenesis is imperative.6-8 Misdiagnosis of the vaginal septa or atresia as an imperforate hymen can lead to significant scarring and stenosis and can make corrective surgical procedures difficult or suboptimal.
| Figure 3. MRI of transverse vaginal septum |
Figure 4. MRI of lower vaginal atresia
Surgical management: hymenectomy
Imperforate hymen is managed surgically with hymenectomy. Repair is generally reserved for the newborn period or, ideally, in adolescence, as at puberty the presence of estrogen aids in surgical repair and healing.5 Simple aspiration of hematocolpos/ mucocolpos can lead to ascending infection, and pyocolpos and should be avoided.6
The goal of hymenectomy is to:
-
open the hymeneal membrane to allow egress of fluid and menstrual flow
-
allow for tampon use
The procedure is relatively straightforward and usually is performed under general anesthesia, although regional anesthesia also is an option.
Steps to the varying hymenectomy incisions
Cruciate incision
1. Incise the hymen at the 2-, 4-, 8-, and 10-o’clock positions into four quadrants.
2. Excise the quadrants along the lateral wall of the vagina.
Elliptical incision
1. Make a circumferential incision with the Bovie electrocautery, incising the hymenal membrane close to the hymenal ring.
U-incision
1. Similar to the elliptical incision, use the Bovie electrocautery to incise the tissue close to the hymenal ring posterior and laterally in a “u” shape.
2. Make a horizontal incision superiorly to remove the extra tissue.
Vertical incision
This incision has been described in cases where there is an attempt to spare the hymen for religious or cultural preference.
1. Make a midline vertical hymenotomy less than 1 cm. Drain the borders of the hymen.
2. Apply suture obliquely to form a circular opening.
References
1. Dominguez C, Rock J, Horowitz I. Surgical conditions of the vagina and urethra. In: TeLinde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1997.
2. Basaran M, Usal D, Aydemir C. Hymen sparing surgery for imperforate hymen: case reports and review of literature. J Pediatr Adolesc Gynecol. 2009;22(4):e61–e64.
Tips to a successful procedure
Ensure adequate suctioning. Before starting the procedure, insert a Foley catheter to completely drain the bladder and delineate the urethra. Making an initial incision into the hymen usually results in the expulsion of the old blood and mucus, which can be very thick and viscous; therefore, it is important to have adequate suction tubing.
Prevent scarring. After evacuating the old blood and mucus, excise the hymeneal membrane with a cruciate incision as is traditionally described. Alternatively, some experts use an elliptical incision or u-incision. (See “Steps to the varying hymenectomy incisions”.) Prevent excision of the hymenal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis and dyspareunia.3
Suturing the mucosal margins is likely unnecessary in adolescent patients. After excision of the hymenal tissue, one option is to suture the mucosal margins of the hymenal ring in an interrupted fashion with a fine, delayed-absorbable suture. Alternatively, at our institution, where we employ the u-incision (FIGURE 5), we assure hemostasis of the mucosal margins and do not suture the margins. Suturing the margins is believed to prevent adherence of the edges; however, in the pubertal girl, adherence is unlikely secondary to estrogen exposure.
Figure 5. Surgical correction with u-incision
Avoid infection; do not irrigate. We do not recommend that you irrigate the vagina and perform unnecessary uterine manipulation, as this may introduce bacteria into the dilated cervix and uterus.3,8
Septate/microperforate/cribiform hymen
These other hymeneal anomalies also may require surgical correction if they become clinically significant. Patients may present with difficulty inserting or removing a tampon, insertional dyspareunia, or incomplete drainage of menstrual blood.6
Imaging is usually not indicated to diagnose these hymenal anomalies, as physical examination will reveal a patent vaginal tract. A moistened Q-tip can be placed into the orifice or behind the septate hymen for confirmation (FIGURE 6).
Surgical correction of a microperforate or cribiform hymen is performed using the same principles as imperforate hymen.
Surgical correction of a septate hymen involves tying and suturing or clamping with a hemostat the upper and lower edges, with the excess hymenal tissue between the sutures then excised.8
Figure 6. Septate hymen
Postop care and follow up
Postoperative analgesia with lidocaine jelly or ice packs is usually sufficient for pain management. Reinforce proper hygienic care measures. At 2- to 3-week follow up, assess the patient for healing and evaluate the size of the hymenal orifice.
Key takeaways
-Differentiating imperforate hymen from low transverse vaginal septum or distal vaginal agenesis prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity.
-With imperforate hymen, examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.
-Pelvic MRI is essential to delineate the anatomy with both vaginal septum and agenesis, for preoperative evaluation of location and thickness of septum as well as measurement of total length of agenesis.
-Hymenectomy is relatively straightforward and may be performed using a cruciate, elliptical, or u-incision.
-Care should be taken to prevent excision of hymeneal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis, and later lead to dyspareunia.
Many gynecologists encounter imperforate hymen, a congenital vaginal anomaly, in general practice. As such, it is important to have a basic understanding of the condition and to be aware of appropriate screening, evaluation, and management. This knowledge will allow you to differentiate imperforate hymen from more complex anomalies—preventing significant morbidity that could result from performing the wrong surgical procedure on this condition—and to provide optimal surgical management.
How often and why does it occur?
Imperforate hymen occurs in approximately 1/1000 newborn girls. It is the most common obstructive anomaly of the female reproductive tract.1,2
The hymen consists of fibrous connective tissue attached to the vaginal wall. In the perinatal period, the hymen serves to separate the vaginal lumen from the urogenital sinus (UGS); this is usually perforated during embryonic life by canalization of the most caudal portion of the vaginal plate at the UGS. This establishes a connection between the lumen of the vaginal canal and the vaginal vestibule.3 Failure of the hymen to perforate completely in the perinatal period can result in varying anomalies, including imperforate (FIGURE 1), microperforate, cribiform, or septated hymen.
Figure 1. Imperforate hymen
How does it present?
Its presentation is variable and frequently asymptomatic in infants and children.4 As a result, the diagnosis is often delayed until puberty.3
In infancy. Newborns typically will present with a hymenal bulge from hydrocolpos or mucocolpos, which result from maternal estrogen secretion on the newborn’s vaginal epithelium.5 This is usually asymptomatic and self limited.
Rarely, large hydrocolpos/mucocolpos may become symptomatic and can lead to urinary obstruction, or they can present as an abdominal mass or intestinal obstruction.4
In adolescence. The majority of adolescents will present with cyclic or persistent pelvic pain and primary amenorrhea. If significant hematometra is present, an abdominal mass also may be palpated. In extreme cases, the patient may present with mass effect symptoms, including back pain, pain with defecation, constipation, nausea and vomiting, urinary retention, or hydronephrosis.6 Retrograde passage of blood into the fallopian tubes can cause hematosalpinx, which can lead to endometriosis and adhesion formation. Blood also may pass freely into the peritoneal cavity, forming hemoperitoneum.3
Related article: Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Imperforate hymen, vaginal septum, or distal vaginal atresia?
When in doubt, refer. Imperforate hymen can be confused with distal vaginal atresia or low transverse vaginal septum. Often, the patient may present with similar signs and symptoms in all 3 cases. Accurately differentiating imperforate hymen from the former two more complex congenital anomalies prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity. As such, it is important to appropriately define the anatomy and refer the complex cases to a specialist comfortable and skilled in managing congenital anomalies, usually a pediatric and adolescent gynecologist or reproductive endocrinologist.
Imperforate hymen
Examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.7 This finding, coupled with a rectal examination and pelvic ultrasonography is usually sufficient to make the diagnosis.6,8 However, magnetic resonance imaging (MRI) of the pelvis should be obtained in cases where the diagnosis is uncertain or the physical exam is more consistent with vaginal septum or agenesis.
Transverse vaginal septum
A reverse septum results from failure of the müllerian duct derivatives and UGS to fuse or canalize. This can occur in the lower, middle, or upper portion of the vagina, and septa may be thick or thin.6 Low transverse septa are more easily confused with imperforate hymen. Examination usually reveals a normal hymen with a short vagina posteriorly. In cases of extreme hematocolpos, vaginal septa also may present with a perineal bulge but, again, this will be posterior to a normal hymen.
Distal vaginal atresia
This condition occurs during embryonic development when the UGS fails to contribute to the lower portion of the vagina (FIGURE 2).5 In cases of distal vaginal atresia there is a lack of vaginal orifice, or only a vaginal dimple may be present.5,6 Rectovaginal examination will reveal a palpable mass if the upper vagina is distended with blood.6
Figure 2. Lower vaginal atresia
MRI is vital to firm diagnosis
In addition to pelvic ultrasonography, pelvic MRI is necessary to delineate the anatomy with both vaginal septum (FIGURE 3) and lower vaginal atresia (FIGURE 4), as preoperative evaluation of location and thickness of a vaginal septum as well as measurement of the total length of agenesis is imperative.6-8 Misdiagnosis of the vaginal septa or atresia as an imperforate hymen can lead to significant scarring and stenosis and can make corrective surgical procedures difficult or suboptimal.
| Figure 3. MRI of transverse vaginal septum |
Figure 4. MRI of lower vaginal atresia
Surgical management: hymenectomy
Imperforate hymen is managed surgically with hymenectomy. Repair is generally reserved for the newborn period or, ideally, in adolescence, as at puberty the presence of estrogen aids in surgical repair and healing.5 Simple aspiration of hematocolpos/ mucocolpos can lead to ascending infection, and pyocolpos and should be avoided.6
The goal of hymenectomy is to:
-
open the hymeneal membrane to allow egress of fluid and menstrual flow
-
allow for tampon use
The procedure is relatively straightforward and usually is performed under general anesthesia, although regional anesthesia also is an option.
Steps to the varying hymenectomy incisions
Cruciate incision
1. Incise the hymen at the 2-, 4-, 8-, and 10-o’clock positions into four quadrants.
2. Excise the quadrants along the lateral wall of the vagina.
Elliptical incision
1. Make a circumferential incision with the Bovie electrocautery, incising the hymenal membrane close to the hymenal ring.
U-incision
1. Similar to the elliptical incision, use the Bovie electrocautery to incise the tissue close to the hymenal ring posterior and laterally in a “u” shape.
2. Make a horizontal incision superiorly to remove the extra tissue.
Vertical incision
This incision has been described in cases where there is an attempt to spare the hymen for religious or cultural preference.
1. Make a midline vertical hymenotomy less than 1 cm. Drain the borders of the hymen.
2. Apply suture obliquely to form a circular opening.
References
1. Dominguez C, Rock J, Horowitz I. Surgical conditions of the vagina and urethra. In: TeLinde’s Operative Gynecology. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1997.
2. Basaran M, Usal D, Aydemir C. Hymen sparing surgery for imperforate hymen: case reports and review of literature. J Pediatr Adolesc Gynecol. 2009;22(4):e61–e64.
Tips to a successful procedure
Ensure adequate suctioning. Before starting the procedure, insert a Foley catheter to completely drain the bladder and delineate the urethra. Making an initial incision into the hymen usually results in the expulsion of the old blood and mucus, which can be very thick and viscous; therefore, it is important to have adequate suction tubing.
Prevent scarring. After evacuating the old blood and mucus, excise the hymeneal membrane with a cruciate incision as is traditionally described. Alternatively, some experts use an elliptical incision or u-incision. (See “Steps to the varying hymenectomy incisions”.) Prevent excision of the hymenal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis and dyspareunia.3
Suturing the mucosal margins is likely unnecessary in adolescent patients. After excision of the hymenal tissue, one option is to suture the mucosal margins of the hymenal ring in an interrupted fashion with a fine, delayed-absorbable suture. Alternatively, at our institution, where we employ the u-incision (FIGURE 5), we assure hemostasis of the mucosal margins and do not suture the margins. Suturing the margins is believed to prevent adherence of the edges; however, in the pubertal girl, adherence is unlikely secondary to estrogen exposure.
Figure 5. Surgical correction with u-incision
Avoid infection; do not irrigate. We do not recommend that you irrigate the vagina and perform unnecessary uterine manipulation, as this may introduce bacteria into the dilated cervix and uterus.3,8
Septate/microperforate/cribiform hymen
These other hymeneal anomalies also may require surgical correction if they become clinically significant. Patients may present with difficulty inserting or removing a tampon, insertional dyspareunia, or incomplete drainage of menstrual blood.6
Imaging is usually not indicated to diagnose these hymenal anomalies, as physical examination will reveal a patent vaginal tract. A moistened Q-tip can be placed into the orifice or behind the septate hymen for confirmation (FIGURE 6).
Surgical correction of a microperforate or cribiform hymen is performed using the same principles as imperforate hymen.
Surgical correction of a septate hymen involves tying and suturing or clamping with a hemostat the upper and lower edges, with the excess hymenal tissue between the sutures then excised.8
Figure 6. Septate hymen
Postop care and follow up
Postoperative analgesia with lidocaine jelly or ice packs is usually sufficient for pain management. Reinforce proper hygienic care measures. At 2- to 3-week follow up, assess the patient for healing and evaluate the size of the hymenal orifice.
Key takeaways
-Differentiating imperforate hymen from low transverse vaginal septum or distal vaginal agenesis prior to surgery is of utmost importance because management is very different, and performing the wrong procedure can result in serious morbidity.
-With imperforate hymen, examination of the external genitalia reveals a perineal bulge secondary to hematocolpos.
-Pelvic MRI is essential to delineate the anatomy with both vaginal septum and agenesis, for preoperative evaluation of location and thickness of septum as well as measurement of total length of agenesis.
-Hymenectomy is relatively straightforward and may be performed using a cruciate, elliptical, or u-incision.
-Care should be taken to prevent excision of hymeneal tissue too close to the vaginal mucosa, as this can lead to scarring and stenosis, and later lead to dyspareunia.
The Affordable Care Act and the drive for electronic health records: Are small practices being squeezed?
Two years ago, I zeroed in on the pressures straining small ObGyn practices in an article entitled, “Is private ObGyn practice on its way out?”1 The pressures haven’t eased in the interim. Today, small practices are still feeling squeezed to keep up with the many demands of modern specialty care. The push for electronic health records (EHRs), in particular, can profoundly affect physicians in private practice.
In this article, I outline some of the challenges facing small practices when they set out to implement EHRs, as well as the potential benefits they stand to gain a little farther down the road. Before we begin, however, let’s look at the latest trends in ObGyn practice, as they are related, in part, to the need to implement EHRs.
The exodus from private practice continues
A 2012 Accenture Physicians Alignment Survey shows an accelerating increase in physician employment. In 2000, 57% of all physicians were in independent practice; by the end of 2013, only 36% of physicians are projected to remain independent.2
The ObGyn specialty is a clear part of this trend, with both seasoned and incoming physicians finding hospital or other employment an attractive alternative to private practice. Fully one-third of ObGyn residents entering practice today sign hospital employment contracts. ObGyns who have made the switch from private to hospital practice, or who have become ObGyn hospitalists, often point to the difficulties of maintaining a solvent private practice, especially given the push toward EHRs and increasing regulatory and administrative burdens, as justification for their move.
The main reasons for the shift to employment. Top concerns influencing physicians’ decisions to opt for employment include:
- business expenses (87%)
- the dominance of managed care (61%)
- the requirement for EHRs (53%)
- the need to maintain and manage staff (53%)
- the increasing number of patients needed to break even (39%).2
A 2008 socioeconomic survey from ACOG revealed that 23.6% of ObGyn practices are solo practices, and 27.1% are single-specialty group practices. Many ObGyns—especially those in solo or small practices—are hesitant to make the large capital investment that is necessary to adopt EHRs.
EHRs offer benefits—and real costs
The system-wide benefits of health information technology (HIT), including EHRs, are many. Insurers stand to save money by reducing unnecessary tests, and patients will benefit from better coordination of their care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices. Instead, physicians face payment cuts from Medicare and private insurance.
Although there’s wide agreement that HIT can improve quality of care and reduce health-care costs, fewer than one-quarter (22%) of office-based physicians had adopted EHRs by 2009. We know the main reasons why:
- upfront cost and maintenance expense
- uncertain return on investment
- fragmented business model in a high proportion of small and solo practices
- changing and inconsistent information technology (IT) systems.
What can a practice expect to fork over?
In 2011, the Agency for Healthcare Research and Quality (AHRQ) found that the “real-life” cost of implementing EHRs “in an average five-physician primary care practice, operating within a large physician network committed to network-wide implementation of electronic health records, is about $162,000, with an additional $85,500 in maintenance expenses during the first year.”3
These figures include an average of 134 hours needed per physician to prepare to use EHRs during patient visits.3
Fleming and colleagues investigated the cost associated with implementing EHRs within 26 primary care practices in Texas. They found the cost to be $32,409 per physician through the first 60 days after the EHR system was launched, with one-time costs for hardware of $25,000 per practice and an additional $7,000 per physician for personal computers, printers, and scanners. The annual cost of software and maintenance was approximately $17,100 per physician.4
Why physicians should hold out for the return on their investment
Despite these considerable expenses, EHRs hold promise over the long term. The Medical Group Management Association reported, through a 2009 survey of about 1,300 primary care and specialty practice members using EHRs, that efficiency gains from the elimination of paper charts, as well as transcription savings, better charge capturing, and reduced billing errors, resulted in a median revenue increase of $49,916 per full-time physician after operating costs.
After 5 years of EHR use, practices reported a median operating margin 10.1% higher than that of practices in the first year of EHR use.5
Trends in the adoption of EHRs
Private practice. An article in Health Affairs showed that, by 2011, only one in six office-based physicians was using an EHR system robust enough to approach “meaningful use”—that is, the use of EHRs to measurably improve the quality of health care.6 These robust systems offered physicians the ability to record information on patient demographics, view laboratory and imaging results, maintain patients’ problem lists, compile clinical notes, and manage prescription ordering. EHR adoption lagged among non−primary care physicians, physicians aged 55 and older, and physicians in small (1–2 providers) practices and physician-owned practices.6 (ObGyns were considered primary care providers in this survey.)
“Big” practice. By comparison, in 2011, 99% of physicians in health maintenance organizations, or HMOs, and 73% in academic health centers and other hospitals used EHR systems.6 The number of physicians in these practice settings is small but growing.
In 2011, only 17% of physicians were in large practices of 10 or more physicians; 40% were in practices of one or two physicians.6
Primary care. These practices lead others in the adoption of EHRs, in part because of federal assistance, including a nationwide system of regional HIT assistance centers established by the Health Information Technology for Economic and Clinical Health (HITECH) Act to help providers located in rural areas participate in the Centers for Medicare and Medicaid Services (CMS) programs in EHR. The goal of these programs is to provide HIT support to at least 100,000 primary care providers, including ObGyns, by 2014.
The numbers cited in the Health Affairs article largely mirror data developed by other research organizations, including the Deloitte Center for Health Solutions.6
The EHR incentive
The drive for EHRs started long before the Affordable Care Act (ACA) was passed in 2010. The US Congress took a first stab at encouraging the health-care community to embrace HIT in 1996, when it passed the Health Insurance Portability and Accountability Act (HIPAA). HIPAA created an electronic data interchange that health plans, health-care clearinghouses, and certain health-care providers, including pharmacists, are required to use for electronic transactions, including:
- claims and encounter information
- payment and remittance advice
- claims status
- eligibility
- enrollment and disenrollment
- referrals and authorizations
- coordination of benefits
- premium payment.
Congress stepped up its game in 2009, when it offered higher Medicare and Medicaid payments to physicians who adopt and “meaningfully use” EHRs. The HITECH Act included $30 billion in new Medicare and Medicaid incentive payments—as much as $44,000 under Medicare and $63,750 under Medicaid—as well as $500 million for states to develop health information exchanges.
The Act also established a government-led process for certification of electronic health records through a $35 billion appropriation for the Office of the National Coordinator for Health IT, housed in CMS.
Other programs designed to encourage use of EHRs
Other federal programs include the Physician Quality Reporting System (PQRS), which, when created in 2006, was a voluntary physician electronic reporting program. Under the ACA, however, it has become a mandate. Starting in 2015, Medicare payments will be reduced for nonparticipating physicians.
The Electronic Prescribing (eRx) Incentive Program, created in 2008 under the Medicare Improvements for Patients and Providers Act, provides incentives for eligible physicians who e-prescribe Medicare Part D medications through a qualified system. This program converted to a penalty program last year for physicians who don’t use eRx.
Grants were also provided under the HITECH Act to fund an HIT infrastructure and low-interest HIT loans. The AHRQ has awarded $300 million in federal grant money to more than 200 projects in 48 states to promote access to and encourage HIT adoption. Over $150 million in Medicaid transformation grants have been awarded to three states and territories for HIT in the Medicaid program under the 2005 Deficit Reduction Act.
The ACA carried these initiatives even further by establishing uniform standards that HIT systems must meet, including:
- automatic reconciliation of electronic fund transfers and HIPAA payment and remittance
- improved claims payment process
- consistent methods of health plan enrollment and claim edits
- simplified and improved routing of health-care transactions
- electronic claims attachments.
Clearly, a lot of effort and taxpayer dollars have been dedicated to drive efficient use of HIT and EHRs in the hopes that they can:
- help make sense of our increasingly fragmented health-care system
- improve patient safety
- increase efficiency
- reduce paperwork
- reduce unnecessary tests
- better coordinate patient care.
To see which providers are cashing in on the government’s incentives for EHRs, see “Some physicians are more likely to seek incentives for meaningful use of EHRs” on page 37.
The long view
HIT and EHRs are here to stay. Products are maturing and improving. Acceptance by large and small practices has gained traction. Are small practices being squeezed? No doubt.
In 2011, I urged all ObGyns—especially those in private practice—to read an article written by President Barack Obama’s health-reform deputies on how physicians can be successful under the ACA.1 It reads, in part:
To realize the full benefits of the Affordable Care Act, physicians will need to embrace rather than resist change. The economic forces put in motion by the Act are likely to lead to vertical organization of providers and accelerate physician employment by hospitals and aggregation into larger physician groups. The most successful physicians will be those who most effectively collaborate with other providers to improve outcomes, care productivity, and patient experience.7
1. DiVenere L, Yates J. Is private ObGyn practice on its way out? OBG Manage. 2011;23(10):42–54.
2. More US doctors leaving private practice due to rising costs and technology mandates, Accenture report finds [news release]. Arlington, Virginia: Accenture Newsroom; October 31, 2012. http://newsroom.accenture.com/news/more-us-doctors-leaving-private-practice-due-to-rising-costs-and-technology-mandates-accenture-report-finds.htm. Accessed June 5, 2013.
3. Study identifies costs of implementing electronic health records in network of physician practices: Research Activities October 2011, No. 374. Rockville, MD: Agency for Healthcare Research and Quality. http://www.ahrq.gov/news/newsletters/research-activities/oct11/1011RA15.html. Accessed June 5, 2013.
5. MGMA survey: Medical groups with EHRs report better financial performance than practices with paper medical records [news release]. New Orleans, Louisiana: Medical Group Management Association; October 25, 2010. http://www.mgma.com/press/default.aspx?id=39824. Accessed June 6, 2013.
8. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicaid Incentive Payments for 2011. GAO-13-146R. December 13, 2012. http://www.gao.gov/products/GAO-13-146R. Accessed June 6, 2013.
9. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicare Incentive Payments for 2011. GAO-12-778R. July 26, 2012. http://www.gao.gov/products/GAO-12-778R. Accessed June 6, 2013.
10. US Department of Health and Human Services. Doctors’ and hospitals’ use of health IT more than doubles since 2012 [news release]. Washington, DC: HHS.gov; May 22, 2013. http://www.hhs.gov/news/press/2013pres/05/20130522a.html. Accessed June 6, 2013.
Two years ago, I zeroed in on the pressures straining small ObGyn practices in an article entitled, “Is private ObGyn practice on its way out?”1 The pressures haven’t eased in the interim. Today, small practices are still feeling squeezed to keep up with the many demands of modern specialty care. The push for electronic health records (EHRs), in particular, can profoundly affect physicians in private practice.
In this article, I outline some of the challenges facing small practices when they set out to implement EHRs, as well as the potential benefits they stand to gain a little farther down the road. Before we begin, however, let’s look at the latest trends in ObGyn practice, as they are related, in part, to the need to implement EHRs.
The exodus from private practice continues
A 2012 Accenture Physicians Alignment Survey shows an accelerating increase in physician employment. In 2000, 57% of all physicians were in independent practice; by the end of 2013, only 36% of physicians are projected to remain independent.2
The ObGyn specialty is a clear part of this trend, with both seasoned and incoming physicians finding hospital or other employment an attractive alternative to private practice. Fully one-third of ObGyn residents entering practice today sign hospital employment contracts. ObGyns who have made the switch from private to hospital practice, or who have become ObGyn hospitalists, often point to the difficulties of maintaining a solvent private practice, especially given the push toward EHRs and increasing regulatory and administrative burdens, as justification for their move.
The main reasons for the shift to employment. Top concerns influencing physicians’ decisions to opt for employment include:
- business expenses (87%)
- the dominance of managed care (61%)
- the requirement for EHRs (53%)
- the need to maintain and manage staff (53%)
- the increasing number of patients needed to break even (39%).2
A 2008 socioeconomic survey from ACOG revealed that 23.6% of ObGyn practices are solo practices, and 27.1% are single-specialty group practices. Many ObGyns—especially those in solo or small practices—are hesitant to make the large capital investment that is necessary to adopt EHRs.
EHRs offer benefits—and real costs
The system-wide benefits of health information technology (HIT), including EHRs, are many. Insurers stand to save money by reducing unnecessary tests, and patients will benefit from better coordination of their care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices. Instead, physicians face payment cuts from Medicare and private insurance.
Although there’s wide agreement that HIT can improve quality of care and reduce health-care costs, fewer than one-quarter (22%) of office-based physicians had adopted EHRs by 2009. We know the main reasons why:
- upfront cost and maintenance expense
- uncertain return on investment
- fragmented business model in a high proportion of small and solo practices
- changing and inconsistent information technology (IT) systems.
What can a practice expect to fork over?
In 2011, the Agency for Healthcare Research and Quality (AHRQ) found that the “real-life” cost of implementing EHRs “in an average five-physician primary care practice, operating within a large physician network committed to network-wide implementation of electronic health records, is about $162,000, with an additional $85,500 in maintenance expenses during the first year.”3
These figures include an average of 134 hours needed per physician to prepare to use EHRs during patient visits.3
Fleming and colleagues investigated the cost associated with implementing EHRs within 26 primary care practices in Texas. They found the cost to be $32,409 per physician through the first 60 days after the EHR system was launched, with one-time costs for hardware of $25,000 per practice and an additional $7,000 per physician for personal computers, printers, and scanners. The annual cost of software and maintenance was approximately $17,100 per physician.4
Why physicians should hold out for the return on their investment
Despite these considerable expenses, EHRs hold promise over the long term. The Medical Group Management Association reported, through a 2009 survey of about 1,300 primary care and specialty practice members using EHRs, that efficiency gains from the elimination of paper charts, as well as transcription savings, better charge capturing, and reduced billing errors, resulted in a median revenue increase of $49,916 per full-time physician after operating costs.
After 5 years of EHR use, practices reported a median operating margin 10.1% higher than that of practices in the first year of EHR use.5
Trends in the adoption of EHRs
Private practice. An article in Health Affairs showed that, by 2011, only one in six office-based physicians was using an EHR system robust enough to approach “meaningful use”—that is, the use of EHRs to measurably improve the quality of health care.6 These robust systems offered physicians the ability to record information on patient demographics, view laboratory and imaging results, maintain patients’ problem lists, compile clinical notes, and manage prescription ordering. EHR adoption lagged among non−primary care physicians, physicians aged 55 and older, and physicians in small (1–2 providers) practices and physician-owned practices.6 (ObGyns were considered primary care providers in this survey.)
“Big” practice. By comparison, in 2011, 99% of physicians in health maintenance organizations, or HMOs, and 73% in academic health centers and other hospitals used EHR systems.6 The number of physicians in these practice settings is small but growing.
In 2011, only 17% of physicians were in large practices of 10 or more physicians; 40% were in practices of one or two physicians.6
Primary care. These practices lead others in the adoption of EHRs, in part because of federal assistance, including a nationwide system of regional HIT assistance centers established by the Health Information Technology for Economic and Clinical Health (HITECH) Act to help providers located in rural areas participate in the Centers for Medicare and Medicaid Services (CMS) programs in EHR. The goal of these programs is to provide HIT support to at least 100,000 primary care providers, including ObGyns, by 2014.
The numbers cited in the Health Affairs article largely mirror data developed by other research organizations, including the Deloitte Center for Health Solutions.6
The EHR incentive
The drive for EHRs started long before the Affordable Care Act (ACA) was passed in 2010. The US Congress took a first stab at encouraging the health-care community to embrace HIT in 1996, when it passed the Health Insurance Portability and Accountability Act (HIPAA). HIPAA created an electronic data interchange that health plans, health-care clearinghouses, and certain health-care providers, including pharmacists, are required to use for electronic transactions, including:
- claims and encounter information
- payment and remittance advice
- claims status
- eligibility
- enrollment and disenrollment
- referrals and authorizations
- coordination of benefits
- premium payment.
Congress stepped up its game in 2009, when it offered higher Medicare and Medicaid payments to physicians who adopt and “meaningfully use” EHRs. The HITECH Act included $30 billion in new Medicare and Medicaid incentive payments—as much as $44,000 under Medicare and $63,750 under Medicaid—as well as $500 million for states to develop health information exchanges.
The Act also established a government-led process for certification of electronic health records through a $35 billion appropriation for the Office of the National Coordinator for Health IT, housed in CMS.
Other programs designed to encourage use of EHRs
Other federal programs include the Physician Quality Reporting System (PQRS), which, when created in 2006, was a voluntary physician electronic reporting program. Under the ACA, however, it has become a mandate. Starting in 2015, Medicare payments will be reduced for nonparticipating physicians.
The Electronic Prescribing (eRx) Incentive Program, created in 2008 under the Medicare Improvements for Patients and Providers Act, provides incentives for eligible physicians who e-prescribe Medicare Part D medications through a qualified system. This program converted to a penalty program last year for physicians who don’t use eRx.
Grants were also provided under the HITECH Act to fund an HIT infrastructure and low-interest HIT loans. The AHRQ has awarded $300 million in federal grant money to more than 200 projects in 48 states to promote access to and encourage HIT adoption. Over $150 million in Medicaid transformation grants have been awarded to three states and territories for HIT in the Medicaid program under the 2005 Deficit Reduction Act.
The ACA carried these initiatives even further by establishing uniform standards that HIT systems must meet, including:
- automatic reconciliation of electronic fund transfers and HIPAA payment and remittance
- improved claims payment process
- consistent methods of health plan enrollment and claim edits
- simplified and improved routing of health-care transactions
- electronic claims attachments.
Clearly, a lot of effort and taxpayer dollars have been dedicated to drive efficient use of HIT and EHRs in the hopes that they can:
- help make sense of our increasingly fragmented health-care system
- improve patient safety
- increase efficiency
- reduce paperwork
- reduce unnecessary tests
- better coordinate patient care.
To see which providers are cashing in on the government’s incentives for EHRs, see “Some physicians are more likely to seek incentives for meaningful use of EHRs” on page 37.
The long view
HIT and EHRs are here to stay. Products are maturing and improving. Acceptance by large and small practices has gained traction. Are small practices being squeezed? No doubt.
In 2011, I urged all ObGyns—especially those in private practice—to read an article written by President Barack Obama’s health-reform deputies on how physicians can be successful under the ACA.1 It reads, in part:
To realize the full benefits of the Affordable Care Act, physicians will need to embrace rather than resist change. The economic forces put in motion by the Act are likely to lead to vertical organization of providers and accelerate physician employment by hospitals and aggregation into larger physician groups. The most successful physicians will be those who most effectively collaborate with other providers to improve outcomes, care productivity, and patient experience.7
Two years ago, I zeroed in on the pressures straining small ObGyn practices in an article entitled, “Is private ObGyn practice on its way out?”1 The pressures haven’t eased in the interim. Today, small practices are still feeling squeezed to keep up with the many demands of modern specialty care. The push for electronic health records (EHRs), in particular, can profoundly affect physicians in private practice.
In this article, I outline some of the challenges facing small practices when they set out to implement EHRs, as well as the potential benefits they stand to gain a little farther down the road. Before we begin, however, let’s look at the latest trends in ObGyn practice, as they are related, in part, to the need to implement EHRs.
The exodus from private practice continues
A 2012 Accenture Physicians Alignment Survey shows an accelerating increase in physician employment. In 2000, 57% of all physicians were in independent practice; by the end of 2013, only 36% of physicians are projected to remain independent.2
The ObGyn specialty is a clear part of this trend, with both seasoned and incoming physicians finding hospital or other employment an attractive alternative to private practice. Fully one-third of ObGyn residents entering practice today sign hospital employment contracts. ObGyns who have made the switch from private to hospital practice, or who have become ObGyn hospitalists, often point to the difficulties of maintaining a solvent private practice, especially given the push toward EHRs and increasing regulatory and administrative burdens, as justification for their move.
The main reasons for the shift to employment. Top concerns influencing physicians’ decisions to opt for employment include:
- business expenses (87%)
- the dominance of managed care (61%)
- the requirement for EHRs (53%)
- the need to maintain and manage staff (53%)
- the increasing number of patients needed to break even (39%).2
A 2008 socioeconomic survey from ACOG revealed that 23.6% of ObGyn practices are solo practices, and 27.1% are single-specialty group practices. Many ObGyns—especially those in solo or small practices—are hesitant to make the large capital investment that is necessary to adopt EHRs.
EHRs offer benefits—and real costs
The system-wide benefits of health information technology (HIT), including EHRs, are many. Insurers stand to save money by reducing unnecessary tests, and patients will benefit from better coordination of their care and fewer medical errors. But these advantages don’t necessarily translate into savings or revenue for physician practices. Instead, physicians face payment cuts from Medicare and private insurance.
Although there’s wide agreement that HIT can improve quality of care and reduce health-care costs, fewer than one-quarter (22%) of office-based physicians had adopted EHRs by 2009. We know the main reasons why:
- upfront cost and maintenance expense
- uncertain return on investment
- fragmented business model in a high proportion of small and solo practices
- changing and inconsistent information technology (IT) systems.
What can a practice expect to fork over?
In 2011, the Agency for Healthcare Research and Quality (AHRQ) found that the “real-life” cost of implementing EHRs “in an average five-physician primary care practice, operating within a large physician network committed to network-wide implementation of electronic health records, is about $162,000, with an additional $85,500 in maintenance expenses during the first year.”3
These figures include an average of 134 hours needed per physician to prepare to use EHRs during patient visits.3
Fleming and colleagues investigated the cost associated with implementing EHRs within 26 primary care practices in Texas. They found the cost to be $32,409 per physician through the first 60 days after the EHR system was launched, with one-time costs for hardware of $25,000 per practice and an additional $7,000 per physician for personal computers, printers, and scanners. The annual cost of software and maintenance was approximately $17,100 per physician.4
Why physicians should hold out for the return on their investment
Despite these considerable expenses, EHRs hold promise over the long term. The Medical Group Management Association reported, through a 2009 survey of about 1,300 primary care and specialty practice members using EHRs, that efficiency gains from the elimination of paper charts, as well as transcription savings, better charge capturing, and reduced billing errors, resulted in a median revenue increase of $49,916 per full-time physician after operating costs.
After 5 years of EHR use, practices reported a median operating margin 10.1% higher than that of practices in the first year of EHR use.5
Trends in the adoption of EHRs
Private practice. An article in Health Affairs showed that, by 2011, only one in six office-based physicians was using an EHR system robust enough to approach “meaningful use”—that is, the use of EHRs to measurably improve the quality of health care.6 These robust systems offered physicians the ability to record information on patient demographics, view laboratory and imaging results, maintain patients’ problem lists, compile clinical notes, and manage prescription ordering. EHR adoption lagged among non−primary care physicians, physicians aged 55 and older, and physicians in small (1–2 providers) practices and physician-owned practices.6 (ObGyns were considered primary care providers in this survey.)
“Big” practice. By comparison, in 2011, 99% of physicians in health maintenance organizations, or HMOs, and 73% in academic health centers and other hospitals used EHR systems.6 The number of physicians in these practice settings is small but growing.
In 2011, only 17% of physicians were in large practices of 10 or more physicians; 40% were in practices of one or two physicians.6
Primary care. These practices lead others in the adoption of EHRs, in part because of federal assistance, including a nationwide system of regional HIT assistance centers established by the Health Information Technology for Economic and Clinical Health (HITECH) Act to help providers located in rural areas participate in the Centers for Medicare and Medicaid Services (CMS) programs in EHR. The goal of these programs is to provide HIT support to at least 100,000 primary care providers, including ObGyns, by 2014.
The numbers cited in the Health Affairs article largely mirror data developed by other research organizations, including the Deloitte Center for Health Solutions.6
The EHR incentive
The drive for EHRs started long before the Affordable Care Act (ACA) was passed in 2010. The US Congress took a first stab at encouraging the health-care community to embrace HIT in 1996, when it passed the Health Insurance Portability and Accountability Act (HIPAA). HIPAA created an electronic data interchange that health plans, health-care clearinghouses, and certain health-care providers, including pharmacists, are required to use for electronic transactions, including:
- claims and encounter information
- payment and remittance advice
- claims status
- eligibility
- enrollment and disenrollment
- referrals and authorizations
- coordination of benefits
- premium payment.
Congress stepped up its game in 2009, when it offered higher Medicare and Medicaid payments to physicians who adopt and “meaningfully use” EHRs. The HITECH Act included $30 billion in new Medicare and Medicaid incentive payments—as much as $44,000 under Medicare and $63,750 under Medicaid—as well as $500 million for states to develop health information exchanges.
The Act also established a government-led process for certification of electronic health records through a $35 billion appropriation for the Office of the National Coordinator for Health IT, housed in CMS.
Other programs designed to encourage use of EHRs
Other federal programs include the Physician Quality Reporting System (PQRS), which, when created in 2006, was a voluntary physician electronic reporting program. Under the ACA, however, it has become a mandate. Starting in 2015, Medicare payments will be reduced for nonparticipating physicians.
The Electronic Prescribing (eRx) Incentive Program, created in 2008 under the Medicare Improvements for Patients and Providers Act, provides incentives for eligible physicians who e-prescribe Medicare Part D medications through a qualified system. This program converted to a penalty program last year for physicians who don’t use eRx.
Grants were also provided under the HITECH Act to fund an HIT infrastructure and low-interest HIT loans. The AHRQ has awarded $300 million in federal grant money to more than 200 projects in 48 states to promote access to and encourage HIT adoption. Over $150 million in Medicaid transformation grants have been awarded to three states and territories for HIT in the Medicaid program under the 2005 Deficit Reduction Act.
The ACA carried these initiatives even further by establishing uniform standards that HIT systems must meet, including:
- automatic reconciliation of electronic fund transfers and HIPAA payment and remittance
- improved claims payment process
- consistent methods of health plan enrollment and claim edits
- simplified and improved routing of health-care transactions
- electronic claims attachments.
Clearly, a lot of effort and taxpayer dollars have been dedicated to drive efficient use of HIT and EHRs in the hopes that they can:
- help make sense of our increasingly fragmented health-care system
- improve patient safety
- increase efficiency
- reduce paperwork
- reduce unnecessary tests
- better coordinate patient care.
To see which providers are cashing in on the government’s incentives for EHRs, see “Some physicians are more likely to seek incentives for meaningful use of EHRs” on page 37.
The long view
HIT and EHRs are here to stay. Products are maturing and improving. Acceptance by large and small practices has gained traction. Are small practices being squeezed? No doubt.
In 2011, I urged all ObGyns—especially those in private practice—to read an article written by President Barack Obama’s health-reform deputies on how physicians can be successful under the ACA.1 It reads, in part:
To realize the full benefits of the Affordable Care Act, physicians will need to embrace rather than resist change. The economic forces put in motion by the Act are likely to lead to vertical organization of providers and accelerate physician employment by hospitals and aggregation into larger physician groups. The most successful physicians will be those who most effectively collaborate with other providers to improve outcomes, care productivity, and patient experience.7
1. DiVenere L, Yates J. Is private ObGyn practice on its way out? OBG Manage. 2011;23(10):42–54.
2. More US doctors leaving private practice due to rising costs and technology mandates, Accenture report finds [news release]. Arlington, Virginia: Accenture Newsroom; October 31, 2012. http://newsroom.accenture.com/news/more-us-doctors-leaving-private-practice-due-to-rising-costs-and-technology-mandates-accenture-report-finds.htm. Accessed June 5, 2013.
3. Study identifies costs of implementing electronic health records in network of physician practices: Research Activities October 2011, No. 374. Rockville, MD: Agency for Healthcare Research and Quality. http://www.ahrq.gov/news/newsletters/research-activities/oct11/1011RA15.html. Accessed June 5, 2013.
5. MGMA survey: Medical groups with EHRs report better financial performance than practices with paper medical records [news release]. New Orleans, Louisiana: Medical Group Management Association; October 25, 2010. http://www.mgma.com/press/default.aspx?id=39824. Accessed June 6, 2013.
8. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicaid Incentive Payments for 2011. GAO-13-146R. December 13, 2012. http://www.gao.gov/products/GAO-13-146R. Accessed June 6, 2013.
9. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicare Incentive Payments for 2011. GAO-12-778R. July 26, 2012. http://www.gao.gov/products/GAO-12-778R. Accessed June 6, 2013.
10. US Department of Health and Human Services. Doctors’ and hospitals’ use of health IT more than doubles since 2012 [news release]. Washington, DC: HHS.gov; May 22, 2013. http://www.hhs.gov/news/press/2013pres/05/20130522a.html. Accessed June 6, 2013.
1. DiVenere L, Yates J. Is private ObGyn practice on its way out? OBG Manage. 2011;23(10):42–54.
2. More US doctors leaving private practice due to rising costs and technology mandates, Accenture report finds [news release]. Arlington, Virginia: Accenture Newsroom; October 31, 2012. http://newsroom.accenture.com/news/more-us-doctors-leaving-private-practice-due-to-rising-costs-and-technology-mandates-accenture-report-finds.htm. Accessed June 5, 2013.
3. Study identifies costs of implementing electronic health records in network of physician practices: Research Activities October 2011, No. 374. Rockville, MD: Agency for Healthcare Research and Quality. http://www.ahrq.gov/news/newsletters/research-activities/oct11/1011RA15.html. Accessed June 5, 2013.
5. MGMA survey: Medical groups with EHRs report better financial performance than practices with paper medical records [news release]. New Orleans, Louisiana: Medical Group Management Association; October 25, 2010. http://www.mgma.com/press/default.aspx?id=39824. Accessed June 6, 2013.
8. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicaid Incentive Payments for 2011. GAO-13-146R. December 13, 2012. http://www.gao.gov/products/GAO-13-146R. Accessed June 6, 2013.
9. US Government Accountability Office. Electronic Health Records: Number and Characteristics of Providers Awarded Medicare Incentive Payments for 2011. GAO-12-778R. July 26, 2012. http://www.gao.gov/products/GAO-12-778R. Accessed June 6, 2013.
10. US Department of Health and Human Services. Doctors’ and hospitals’ use of health IT more than doubles since 2012 [news release]. Washington, DC: HHS.gov; May 22, 2013. http://www.hhs.gov/news/press/2013pres/05/20130522a.html. Accessed June 6, 2013.
How to manage emergencies associated with tocolysis for preterm labor
CASE 1: Preterm labor with cervical changes
Ms. M, a 42-year-old woman pregnant with her second child, begins having contractions at 30 weeks’ gestation. Examination reveals that her cervix is dilated 2 cm and effaced 50%. She is given subcutaneous terbutaline to suppress her contractions. Thirty minutes later, she complains of shortness of breath and chest pain. An electrocardiogram reveals depression of the ST segment, and a chest radiograph shows mild pulmonary edema.
How should her symptoms be managed?
Preterm labor precedes delivery in about 50% of preterm births. Approximately 33% of women who have preterm labor will experience spontaneous resolution, and more than 50% of women who have preterm labor will deliver at term. Although the use of tocolytic therapy has proved to be effective at temporarily suppressing uterine activity, it has not been shown to delay delivery for more than a few hours or days.1
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of tocolytics only when a delay in labor for approximately 48 hours would improve outcome. Therefore, tocolytic therapy should be reserved for the following circumstances:
- to stop the progress of labor long enough to administer antenatal corticosteroid therapy
- to prolong pregnancy when there is an underlying self-limiting condition that can cause labor, such as pyelonephritis
- to provide time for safe transport to a facility with a higher level of neonatal care.2
Tocolytics are generally not indicated before the fetus is viable, although we lack data from randomized, controlled trials to support a specific recommendation. The approach is clearer when the fetus is near the upper limits of viability. Most studies suggest that 34 weeks’ gestation is the threshold at which the perinatal morbidity and mortality associated with delivery are too low to justify the cost and potential complications of tocolysis.3
Women who experience preterm labor without cervical changes generally should not be treated with tocolytics.2 Contraindications to tocolytic therapy include:
- lethal fetal anomaly
- nonreassuring fetal status
- maternal disease
- maternal hemorrhage with hemodynamic instability.
Beta-adrenergic agonists carry many risks
These agents have been studied in several randomized, controlled trials. Although ritodrine was approved as tocolytic therapy by the US Food and Drug Administration (FDA), it has since been removed from the US market. Terbutaline is still available but lacks FDA approval as a tocolytic.
Maternal side effects associated with beta-adrenergic agonists are thought to arise from stimulation of the beta-1 and beta-2 adrenergic receptors. Stimulation of the former increases maternal heart rate and stroke volume, whereas stimulation of the beta-2 adrenergic receptors causes the relaxation of smooth muscle, including the muscles of the myometrium, blood vessels, and bronchial tree. The resulting symptoms may include maternal tachycardia, cardiac arrhythmias, palpitations, and metabolic aberrations (including hyperglycemia, hypokalemia, and hypotension). Common symptoms associated with the administration of a beta-adrenergic agonist include tremor, shortness of breath, and chest discomfort.4 Although pulmonary edema and myocardial ischemia are uncommon, they can occur even when there is no history of underlying maternal disease.
Terbutaline has been linked to maternal deaths
Sixteen maternal deaths were reported following initial marketing of terbutaline in 1976 until 2009. Three of the 16 cases involved outpatient use of terbutaline administered by a subcutaneous pump, and nine cases involved use of oral terbutaline alone or in addition to subcutaneous or IV terbutaline. In addition, 12 cases of serious maternal cardiovascular events were reported in association with terbutaline. These events included cardiac arrhythmias, myocardial infarction, pulmonary edema, hypertension, and tachycardia.
Because of these events, the FDA issued a black box warning for terbutaline that prohibits its use in the treatment of preterm labor for longer than 48 to 72 hours in the inpatient or outpatient setting because of the potential for serious maternal heart problems and death.5 Oral terbutaline should be avoided entirely in the prevention and treatment of preterm labor. However, the use of terbutaline for the management of acute tachysystole with an abnormal fetal heart-rate (FHR) pattern remains a reasonable course of treatment.6
Fetal tachycardia is the most common side effect of beta-adrenergic receptor agonists. For this reason, use of these drugs is not recommended when changes in FHR may be the first sign of fetal compromise, such as in patients with hemorrhage or infection. Neonatal hypoglycemia may also occur if maternal hyperglycemia is not controlled.7
Case 1 Resolved
Terbutaline is discontinued, and the patient’s pulmonary edema is treated with a single dose of furosemide. Electrolyte abnormalities resolve with discontinuation of medication. The patient stabilizes. Once her cardiorespiratory status improves, her contractions lessen and the cervix remains unchanged. She requires no further tocolysis and is discharged home. She presents again at 38 weeks in spontaneous labor.
CASE 2: Preterm labor treated with indomethacin
Ms. J, age 23, is 26 weeks’ pregnant with her first child. When she experienced preterm labor at 24.5 weeks’ gestation, she was given indomethacin. Now, ultrasonographic imaging reveals decreased amniotic fluid volume.
How should she be managed?
Indomethacin is a cyclooxygenase (COX) inhibitor. These drugs reduce prostaglandin production through the general inhibition of cyclooxygenase or by a specific receptor.8 Indomethacin is the most commonly used tocolytic in this class. It is a nonspecific COX inhibitor, as opposed to a COX-2 inhibitor. The latter has been associated with serious adverse outcomes in the nonobstetric population. COX-2 inhibitors now carry a black box warning or are no longer available.
Maternal contraindications for COX inhibitors include asthma, bleeding disorders, and significant renal dysfunction.
Although maternal side effects with COX inhibitors are usually mild, fetal side effects may be serious enough to cause perinatal morbidity or death.9
How indomethacin can lead to oligohydramnios
Maternal administration of indomethacin or ibuprofen can reduce fetal urine output and decrease the volume of amniotic fluid. In most cases, oligohydramnios occurs when indomethacin or ibuprofen has been given for more than 72 hours. For this reason, long-term use of a COX inhibitor should be accompanied by frequent monitoring of amniotic fluid volume by ultrasonography.
The most serious fetal complication associated with prolonged indomethacin administration (longer than 72 hours) is premature constriction of the ductus arteriosus. Ductal constriction appears to be contingent on gestational age. It has been described as early as 24 weeks’ gestation but is most common after 31 or 32 weeks. Therefore, indomethacin is not recommended for use after 32 weeks’ gestation.10
CASE 2 Resolved
The indomethacin is discontinued as soon as the decreased amniotic fluid is noted. The fluid volume returns to normal over the next 3 to 5 days. Because of the early gestational age, nifedipine is given to suppress contractions, and the patient has no further complications.
CASE 3: Preterm labor and magnesium intoxication
Ms. K experiences contractions and rapid cervical change at 32 weeks’ gestation. She is given magnesium for the preterm labor and fetal neuroprophylaxis, with nifedipine, a calcium-channel blocker, added as second-line tocolysis. Approximately 8 hours later, she reports difficulty breathing and moving.
How should her obstetrician proceed?
Calcium-channel blockers such as nifedipine are used for acute and maintenance tocolysis. This class of drugs is often selected for its relative ease of use and safety, as it has few maternal and fetal side effects. However, concomitant use of a calcium-channel blocker and magnesium sulfate can sometimes lead to neuromuscular blockade and significant respiratory depression, even necessitating mechanical ventilation.9 Treatment of these effects includes IV administration of 10% calcium gluconate (5–10 mEq), which usually reverses respiratory depression and heart block caused by magnesium intoxication. In extreme cases, peritoneal dialysis or hemodialysis may be required.
CASE 3 Resolved
The patient is given 10% calcium gluconate in the dosage described above, and she stabilizes. However, her contractions continue and she delivers at 32 weeks’ gestation. The infant does well in the NICU.
CASE 4: Preterm labor in a woman with kidney dysfunction
Ms. F, age 40, presents at 30 weeks’ gestation with regular contractions and cervical dilation of more than 3 cm. She also reports a history of kidney disease.
What steps are recommended prior to the initiation of magnesium therapy?
Magnesium sulfate has been used for more than 40 years to treat preterm labor and is still considered a first-line therapy in many centers. Although maternal side effects usually are mild, an adverse event may occur if the patient is not monitored carefully. An absence of deep-tendon reflexes should alert the clinician that magnesium levels need to be measured. Reflexes usually are lost at a serum level of 10 mEq/L or higher. When the magnesium level exceeds 13 mEq/L, cardiac arrest is a risk. IV calcium should be administered immediately in such patients.
Magnesium should be used with caution in patients with myocardial compromise. Because magnesium is eliminated by the kidneys, women with impaired renal function may experience magnesium toxicity at normal doses. If a patient has a creatinine level above 1 mg/dL, consider alternative treatment for her preterm labor. If magnesium is given, the normal loading dose (4–6 g) is appropriate, but the maintenance dose should be reduced.11
Fetal effects of magnesium sulfate
Recent studies indicate that predelivery magnesium may offer fetal neuroprotection. The minimum duration of administration for such neuroprotection is unknown but is less than 24 hours.8
Although magnesium can alter FHR patterns slightly, these changes are not clinically significant. Magnesium can also cause mild neonatal suppression at the time of delivery, but its effects quickly resolve with appropriate neonatal resuscitation. Long-term (>5 days) therapy is not recommended.
In May 2013, the FDA issued a warning about the risk of neonatal complications with long-term maternal magnesium administration. These complications include osteopenia, low calcium, and bone fracture. The pregnancy category for magnesium sulfate will be changed from “A” to “D” because of these teratogenic effects.12
CASE 4 Resolved
Because magnesium is mainly cleared by renal excretion, the clinician administers the medication with caution in this patient with reduced renal function. The clinician administers the same 4- to 6-g bolus that would be given a patient with normal kidney function, but the maintenance dose is reduced to 1 g. Magnesium levels are obtained every 12 hours or when clinically indicated.
Bottom line: Be ready to act
The short-term use of tocolytic therapy usually is not associated with maternal or fetal complications. After initial administration, maintenance tocolytic therapy probably does not prolong gestation.
Given the potential for harm without additional fetal benefit associated with extended therapy, I recommend that clinicians follow current clinical guidelines from ACOG for use of tocolytic agents. In the process, be vigilant for complications and be ready to act appropriately. Keep maternal and fetal conditions in mind when selecting a tocolytic agent.
9. US Food and Drug Administration. Terbutaline: Label Change—Warnings Against Use for Treatment of Preterm Labor. Published February 17, 2011. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyalertsforHumanMedicalProducts/ucm243843.htm. Accessed June 17, 2013.
12. US Food and Drug Administration. Magnesium Sulfate: Drug Safety Communication—Recommendation against Prolonged Use in Preterm Labor. Published May 30, 2013. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm354603.htm. Accessed June 17, 2013.
CASE 1: Preterm labor with cervical changes
Ms. M, a 42-year-old woman pregnant with her second child, begins having contractions at 30 weeks’ gestation. Examination reveals that her cervix is dilated 2 cm and effaced 50%. She is given subcutaneous terbutaline to suppress her contractions. Thirty minutes later, she complains of shortness of breath and chest pain. An electrocardiogram reveals depression of the ST segment, and a chest radiograph shows mild pulmonary edema.
How should her symptoms be managed?
Preterm labor precedes delivery in about 50% of preterm births. Approximately 33% of women who have preterm labor will experience spontaneous resolution, and more than 50% of women who have preterm labor will deliver at term. Although the use of tocolytic therapy has proved to be effective at temporarily suppressing uterine activity, it has not been shown to delay delivery for more than a few hours or days.1
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of tocolytics only when a delay in labor for approximately 48 hours would improve outcome. Therefore, tocolytic therapy should be reserved for the following circumstances:
- to stop the progress of labor long enough to administer antenatal corticosteroid therapy
- to prolong pregnancy when there is an underlying self-limiting condition that can cause labor, such as pyelonephritis
- to provide time for safe transport to a facility with a higher level of neonatal care.2
Tocolytics are generally not indicated before the fetus is viable, although we lack data from randomized, controlled trials to support a specific recommendation. The approach is clearer when the fetus is near the upper limits of viability. Most studies suggest that 34 weeks’ gestation is the threshold at which the perinatal morbidity and mortality associated with delivery are too low to justify the cost and potential complications of tocolysis.3
Women who experience preterm labor without cervical changes generally should not be treated with tocolytics.2 Contraindications to tocolytic therapy include:
- lethal fetal anomaly
- nonreassuring fetal status
- maternal disease
- maternal hemorrhage with hemodynamic instability.
Beta-adrenergic agonists carry many risks
These agents have been studied in several randomized, controlled trials. Although ritodrine was approved as tocolytic therapy by the US Food and Drug Administration (FDA), it has since been removed from the US market. Terbutaline is still available but lacks FDA approval as a tocolytic.
Maternal side effects associated with beta-adrenergic agonists are thought to arise from stimulation of the beta-1 and beta-2 adrenergic receptors. Stimulation of the former increases maternal heart rate and stroke volume, whereas stimulation of the beta-2 adrenergic receptors causes the relaxation of smooth muscle, including the muscles of the myometrium, blood vessels, and bronchial tree. The resulting symptoms may include maternal tachycardia, cardiac arrhythmias, palpitations, and metabolic aberrations (including hyperglycemia, hypokalemia, and hypotension). Common symptoms associated with the administration of a beta-adrenergic agonist include tremor, shortness of breath, and chest discomfort.4 Although pulmonary edema and myocardial ischemia are uncommon, they can occur even when there is no history of underlying maternal disease.
Terbutaline has been linked to maternal deaths
Sixteen maternal deaths were reported following initial marketing of terbutaline in 1976 until 2009. Three of the 16 cases involved outpatient use of terbutaline administered by a subcutaneous pump, and nine cases involved use of oral terbutaline alone or in addition to subcutaneous or IV terbutaline. In addition, 12 cases of serious maternal cardiovascular events were reported in association with terbutaline. These events included cardiac arrhythmias, myocardial infarction, pulmonary edema, hypertension, and tachycardia.
Because of these events, the FDA issued a black box warning for terbutaline that prohibits its use in the treatment of preterm labor for longer than 48 to 72 hours in the inpatient or outpatient setting because of the potential for serious maternal heart problems and death.5 Oral terbutaline should be avoided entirely in the prevention and treatment of preterm labor. However, the use of terbutaline for the management of acute tachysystole with an abnormal fetal heart-rate (FHR) pattern remains a reasonable course of treatment.6
Fetal tachycardia is the most common side effect of beta-adrenergic receptor agonists. For this reason, use of these drugs is not recommended when changes in FHR may be the first sign of fetal compromise, such as in patients with hemorrhage or infection. Neonatal hypoglycemia may also occur if maternal hyperglycemia is not controlled.7
Case 1 Resolved
Terbutaline is discontinued, and the patient’s pulmonary edema is treated with a single dose of furosemide. Electrolyte abnormalities resolve with discontinuation of medication. The patient stabilizes. Once her cardiorespiratory status improves, her contractions lessen and the cervix remains unchanged. She requires no further tocolysis and is discharged home. She presents again at 38 weeks in spontaneous labor.
CASE 2: Preterm labor treated with indomethacin
Ms. J, age 23, is 26 weeks’ pregnant with her first child. When she experienced preterm labor at 24.5 weeks’ gestation, she was given indomethacin. Now, ultrasonographic imaging reveals decreased amniotic fluid volume.
How should she be managed?
Indomethacin is a cyclooxygenase (COX) inhibitor. These drugs reduce prostaglandin production through the general inhibition of cyclooxygenase or by a specific receptor.8 Indomethacin is the most commonly used tocolytic in this class. It is a nonspecific COX inhibitor, as opposed to a COX-2 inhibitor. The latter has been associated with serious adverse outcomes in the nonobstetric population. COX-2 inhibitors now carry a black box warning or are no longer available.
Maternal contraindications for COX inhibitors include asthma, bleeding disorders, and significant renal dysfunction.
Although maternal side effects with COX inhibitors are usually mild, fetal side effects may be serious enough to cause perinatal morbidity or death.9
How indomethacin can lead to oligohydramnios
Maternal administration of indomethacin or ibuprofen can reduce fetal urine output and decrease the volume of amniotic fluid. In most cases, oligohydramnios occurs when indomethacin or ibuprofen has been given for more than 72 hours. For this reason, long-term use of a COX inhibitor should be accompanied by frequent monitoring of amniotic fluid volume by ultrasonography.
The most serious fetal complication associated with prolonged indomethacin administration (longer than 72 hours) is premature constriction of the ductus arteriosus. Ductal constriction appears to be contingent on gestational age. It has been described as early as 24 weeks’ gestation but is most common after 31 or 32 weeks. Therefore, indomethacin is not recommended for use after 32 weeks’ gestation.10
CASE 2 Resolved
The indomethacin is discontinued as soon as the decreased amniotic fluid is noted. The fluid volume returns to normal over the next 3 to 5 days. Because of the early gestational age, nifedipine is given to suppress contractions, and the patient has no further complications.
CASE 3: Preterm labor and magnesium intoxication
Ms. K experiences contractions and rapid cervical change at 32 weeks’ gestation. She is given magnesium for the preterm labor and fetal neuroprophylaxis, with nifedipine, a calcium-channel blocker, added as second-line tocolysis. Approximately 8 hours later, she reports difficulty breathing and moving.
How should her obstetrician proceed?
Calcium-channel blockers such as nifedipine are used for acute and maintenance tocolysis. This class of drugs is often selected for its relative ease of use and safety, as it has few maternal and fetal side effects. However, concomitant use of a calcium-channel blocker and magnesium sulfate can sometimes lead to neuromuscular blockade and significant respiratory depression, even necessitating mechanical ventilation.9 Treatment of these effects includes IV administration of 10% calcium gluconate (5–10 mEq), which usually reverses respiratory depression and heart block caused by magnesium intoxication. In extreme cases, peritoneal dialysis or hemodialysis may be required.
CASE 3 Resolved
The patient is given 10% calcium gluconate in the dosage described above, and she stabilizes. However, her contractions continue and she delivers at 32 weeks’ gestation. The infant does well in the NICU.
CASE 4: Preterm labor in a woman with kidney dysfunction
Ms. F, age 40, presents at 30 weeks’ gestation with regular contractions and cervical dilation of more than 3 cm. She also reports a history of kidney disease.
What steps are recommended prior to the initiation of magnesium therapy?
Magnesium sulfate has been used for more than 40 years to treat preterm labor and is still considered a first-line therapy in many centers. Although maternal side effects usually are mild, an adverse event may occur if the patient is not monitored carefully. An absence of deep-tendon reflexes should alert the clinician that magnesium levels need to be measured. Reflexes usually are lost at a serum level of 10 mEq/L or higher. When the magnesium level exceeds 13 mEq/L, cardiac arrest is a risk. IV calcium should be administered immediately in such patients.
Magnesium should be used with caution in patients with myocardial compromise. Because magnesium is eliminated by the kidneys, women with impaired renal function may experience magnesium toxicity at normal doses. If a patient has a creatinine level above 1 mg/dL, consider alternative treatment for her preterm labor. If magnesium is given, the normal loading dose (4–6 g) is appropriate, but the maintenance dose should be reduced.11
Fetal effects of magnesium sulfate
Recent studies indicate that predelivery magnesium may offer fetal neuroprotection. The minimum duration of administration for such neuroprotection is unknown but is less than 24 hours.8
Although magnesium can alter FHR patterns slightly, these changes are not clinically significant. Magnesium can also cause mild neonatal suppression at the time of delivery, but its effects quickly resolve with appropriate neonatal resuscitation. Long-term (>5 days) therapy is not recommended.
In May 2013, the FDA issued a warning about the risk of neonatal complications with long-term maternal magnesium administration. These complications include osteopenia, low calcium, and bone fracture. The pregnancy category for magnesium sulfate will be changed from “A” to “D” because of these teratogenic effects.12
CASE 4 Resolved
Because magnesium is mainly cleared by renal excretion, the clinician administers the medication with caution in this patient with reduced renal function. The clinician administers the same 4- to 6-g bolus that would be given a patient with normal kidney function, but the maintenance dose is reduced to 1 g. Magnesium levels are obtained every 12 hours or when clinically indicated.
Bottom line: Be ready to act
The short-term use of tocolytic therapy usually is not associated with maternal or fetal complications. After initial administration, maintenance tocolytic therapy probably does not prolong gestation.
Given the potential for harm without additional fetal benefit associated with extended therapy, I recommend that clinicians follow current clinical guidelines from ACOG for use of tocolytic agents. In the process, be vigilant for complications and be ready to act appropriately. Keep maternal and fetal conditions in mind when selecting a tocolytic agent.
CASE 1: Preterm labor with cervical changes
Ms. M, a 42-year-old woman pregnant with her second child, begins having contractions at 30 weeks’ gestation. Examination reveals that her cervix is dilated 2 cm and effaced 50%. She is given subcutaneous terbutaline to suppress her contractions. Thirty minutes later, she complains of shortness of breath and chest pain. An electrocardiogram reveals depression of the ST segment, and a chest radiograph shows mild pulmonary edema.
How should her symptoms be managed?
Preterm labor precedes delivery in about 50% of preterm births. Approximately 33% of women who have preterm labor will experience spontaneous resolution, and more than 50% of women who have preterm labor will deliver at term. Although the use of tocolytic therapy has proved to be effective at temporarily suppressing uterine activity, it has not been shown to delay delivery for more than a few hours or days.1
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of tocolytics only when a delay in labor for approximately 48 hours would improve outcome. Therefore, tocolytic therapy should be reserved for the following circumstances:
- to stop the progress of labor long enough to administer antenatal corticosteroid therapy
- to prolong pregnancy when there is an underlying self-limiting condition that can cause labor, such as pyelonephritis
- to provide time for safe transport to a facility with a higher level of neonatal care.2
Tocolytics are generally not indicated before the fetus is viable, although we lack data from randomized, controlled trials to support a specific recommendation. The approach is clearer when the fetus is near the upper limits of viability. Most studies suggest that 34 weeks’ gestation is the threshold at which the perinatal morbidity and mortality associated with delivery are too low to justify the cost and potential complications of tocolysis.3
Women who experience preterm labor without cervical changes generally should not be treated with tocolytics.2 Contraindications to tocolytic therapy include:
- lethal fetal anomaly
- nonreassuring fetal status
- maternal disease
- maternal hemorrhage with hemodynamic instability.
Beta-adrenergic agonists carry many risks
These agents have been studied in several randomized, controlled trials. Although ritodrine was approved as tocolytic therapy by the US Food and Drug Administration (FDA), it has since been removed from the US market. Terbutaline is still available but lacks FDA approval as a tocolytic.
Maternal side effects associated with beta-adrenergic agonists are thought to arise from stimulation of the beta-1 and beta-2 adrenergic receptors. Stimulation of the former increases maternal heart rate and stroke volume, whereas stimulation of the beta-2 adrenergic receptors causes the relaxation of smooth muscle, including the muscles of the myometrium, blood vessels, and bronchial tree. The resulting symptoms may include maternal tachycardia, cardiac arrhythmias, palpitations, and metabolic aberrations (including hyperglycemia, hypokalemia, and hypotension). Common symptoms associated with the administration of a beta-adrenergic agonist include tremor, shortness of breath, and chest discomfort.4 Although pulmonary edema and myocardial ischemia are uncommon, they can occur even when there is no history of underlying maternal disease.
Terbutaline has been linked to maternal deaths
Sixteen maternal deaths were reported following initial marketing of terbutaline in 1976 until 2009. Three of the 16 cases involved outpatient use of terbutaline administered by a subcutaneous pump, and nine cases involved use of oral terbutaline alone or in addition to subcutaneous or IV terbutaline. In addition, 12 cases of serious maternal cardiovascular events were reported in association with terbutaline. These events included cardiac arrhythmias, myocardial infarction, pulmonary edema, hypertension, and tachycardia.
Because of these events, the FDA issued a black box warning for terbutaline that prohibits its use in the treatment of preterm labor for longer than 48 to 72 hours in the inpatient or outpatient setting because of the potential for serious maternal heart problems and death.5 Oral terbutaline should be avoided entirely in the prevention and treatment of preterm labor. However, the use of terbutaline for the management of acute tachysystole with an abnormal fetal heart-rate (FHR) pattern remains a reasonable course of treatment.6
Fetal tachycardia is the most common side effect of beta-adrenergic receptor agonists. For this reason, use of these drugs is not recommended when changes in FHR may be the first sign of fetal compromise, such as in patients with hemorrhage or infection. Neonatal hypoglycemia may also occur if maternal hyperglycemia is not controlled.7
Case 1 Resolved
Terbutaline is discontinued, and the patient’s pulmonary edema is treated with a single dose of furosemide. Electrolyte abnormalities resolve with discontinuation of medication. The patient stabilizes. Once her cardiorespiratory status improves, her contractions lessen and the cervix remains unchanged. She requires no further tocolysis and is discharged home. She presents again at 38 weeks in spontaneous labor.
CASE 2: Preterm labor treated with indomethacin
Ms. J, age 23, is 26 weeks’ pregnant with her first child. When she experienced preterm labor at 24.5 weeks’ gestation, she was given indomethacin. Now, ultrasonographic imaging reveals decreased amniotic fluid volume.
How should she be managed?
Indomethacin is a cyclooxygenase (COX) inhibitor. These drugs reduce prostaglandin production through the general inhibition of cyclooxygenase or by a specific receptor.8 Indomethacin is the most commonly used tocolytic in this class. It is a nonspecific COX inhibitor, as opposed to a COX-2 inhibitor. The latter has been associated with serious adverse outcomes in the nonobstetric population. COX-2 inhibitors now carry a black box warning or are no longer available.
Maternal contraindications for COX inhibitors include asthma, bleeding disorders, and significant renal dysfunction.
Although maternal side effects with COX inhibitors are usually mild, fetal side effects may be serious enough to cause perinatal morbidity or death.9
How indomethacin can lead to oligohydramnios
Maternal administration of indomethacin or ibuprofen can reduce fetal urine output and decrease the volume of amniotic fluid. In most cases, oligohydramnios occurs when indomethacin or ibuprofen has been given for more than 72 hours. For this reason, long-term use of a COX inhibitor should be accompanied by frequent monitoring of amniotic fluid volume by ultrasonography.
The most serious fetal complication associated with prolonged indomethacin administration (longer than 72 hours) is premature constriction of the ductus arteriosus. Ductal constriction appears to be contingent on gestational age. It has been described as early as 24 weeks’ gestation but is most common after 31 or 32 weeks. Therefore, indomethacin is not recommended for use after 32 weeks’ gestation.10
CASE 2 Resolved
The indomethacin is discontinued as soon as the decreased amniotic fluid is noted. The fluid volume returns to normal over the next 3 to 5 days. Because of the early gestational age, nifedipine is given to suppress contractions, and the patient has no further complications.
CASE 3: Preterm labor and magnesium intoxication
Ms. K experiences contractions and rapid cervical change at 32 weeks’ gestation. She is given magnesium for the preterm labor and fetal neuroprophylaxis, with nifedipine, a calcium-channel blocker, added as second-line tocolysis. Approximately 8 hours later, she reports difficulty breathing and moving.
How should her obstetrician proceed?
Calcium-channel blockers such as nifedipine are used for acute and maintenance tocolysis. This class of drugs is often selected for its relative ease of use and safety, as it has few maternal and fetal side effects. However, concomitant use of a calcium-channel blocker and magnesium sulfate can sometimes lead to neuromuscular blockade and significant respiratory depression, even necessitating mechanical ventilation.9 Treatment of these effects includes IV administration of 10% calcium gluconate (5–10 mEq), which usually reverses respiratory depression and heart block caused by magnesium intoxication. In extreme cases, peritoneal dialysis or hemodialysis may be required.
CASE 3 Resolved
The patient is given 10% calcium gluconate in the dosage described above, and she stabilizes. However, her contractions continue and she delivers at 32 weeks’ gestation. The infant does well in the NICU.
CASE 4: Preterm labor in a woman with kidney dysfunction
Ms. F, age 40, presents at 30 weeks’ gestation with regular contractions and cervical dilation of more than 3 cm. She also reports a history of kidney disease.
What steps are recommended prior to the initiation of magnesium therapy?
Magnesium sulfate has been used for more than 40 years to treat preterm labor and is still considered a first-line therapy in many centers. Although maternal side effects usually are mild, an adverse event may occur if the patient is not monitored carefully. An absence of deep-tendon reflexes should alert the clinician that magnesium levels need to be measured. Reflexes usually are lost at a serum level of 10 mEq/L or higher. When the magnesium level exceeds 13 mEq/L, cardiac arrest is a risk. IV calcium should be administered immediately in such patients.
Magnesium should be used with caution in patients with myocardial compromise. Because magnesium is eliminated by the kidneys, women with impaired renal function may experience magnesium toxicity at normal doses. If a patient has a creatinine level above 1 mg/dL, consider alternative treatment for her preterm labor. If magnesium is given, the normal loading dose (4–6 g) is appropriate, but the maintenance dose should be reduced.11
Fetal effects of magnesium sulfate
Recent studies indicate that predelivery magnesium may offer fetal neuroprotection. The minimum duration of administration for such neuroprotection is unknown but is less than 24 hours.8
Although magnesium can alter FHR patterns slightly, these changes are not clinically significant. Magnesium can also cause mild neonatal suppression at the time of delivery, but its effects quickly resolve with appropriate neonatal resuscitation. Long-term (>5 days) therapy is not recommended.
In May 2013, the FDA issued a warning about the risk of neonatal complications with long-term maternal magnesium administration. These complications include osteopenia, low calcium, and bone fracture. The pregnancy category for magnesium sulfate will be changed from “A” to “D” because of these teratogenic effects.12
CASE 4 Resolved
Because magnesium is mainly cleared by renal excretion, the clinician administers the medication with caution in this patient with reduced renal function. The clinician administers the same 4- to 6-g bolus that would be given a patient with normal kidney function, but the maintenance dose is reduced to 1 g. Magnesium levels are obtained every 12 hours or when clinically indicated.
Bottom line: Be ready to act
The short-term use of tocolytic therapy usually is not associated with maternal or fetal complications. After initial administration, maintenance tocolytic therapy probably does not prolong gestation.
Given the potential for harm without additional fetal benefit associated with extended therapy, I recommend that clinicians follow current clinical guidelines from ACOG for use of tocolytic agents. In the process, be vigilant for complications and be ready to act appropriately. Keep maternal and fetal conditions in mind when selecting a tocolytic agent.
9. US Food and Drug Administration. Terbutaline: Label Change—Warnings Against Use for Treatment of Preterm Labor. Published February 17, 2011. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyalertsforHumanMedicalProducts/ucm243843.htm. Accessed June 17, 2013.
12. US Food and Drug Administration. Magnesium Sulfate: Drug Safety Communication—Recommendation against Prolonged Use in Preterm Labor. Published May 30, 2013. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm354603.htm. Accessed June 17, 2013.
9. US Food and Drug Administration. Terbutaline: Label Change—Warnings Against Use for Treatment of Preterm Labor. Published February 17, 2011. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyalertsforHumanMedicalProducts/ucm243843.htm. Accessed June 17, 2013.
12. US Food and Drug Administration. Magnesium Sulfate: Drug Safety Communication—Recommendation against Prolonged Use in Preterm Labor. Published May 30, 2013. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm354603.htm. Accessed June 17, 2013.
Beta-adrenergic agonists carry many risks
Fetal effects of magnesium sulfate
Do cosmetic breast implants hinder the detection of malignancy and reduce breast cancer–specific survival?
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.
Most epidemiologic studies have found no elevated risk of breast cancer among women who undergo cosmetic breast augmentation. However, there is concern that implants, which are radio-opaque, may limit our ability to diagnose malignancies at an early stage using screening mammography.
In this study, investigators compared the stage distribution of breast cancers at diagnosis and documented breast cancer–specific survival among women with and without cosmetic breast implants. Twelve cross-sectional studies published after 2000 in the United States had evaluated stage distribution of breast cancer among women with and without cosmetic implants. As stated above, investigators found an elevated risk of nonlocalized breast cancer among women with implants in their meta-analysis of these studies (OR, 1.26), but this elevated risk did not achieve statistical significance. A second analysis of five studies found an elevated risk of breast cancer–specific mortality (OR, 1.38), compared with the general population (no implants), which did achieve significance.
MRI may be helpful—but is the expense justified?
More than 300,000 women underwent cosmetic breast augmentation in 2011 in the United States, an increase of roughly 800% since the early 1990s. The impaired visualization of breast tissue via mammography in these women ranges from 22% to 83%. In addition, the implants limit compression of the breasts during mammography, and capsular contraction further contributes to this problem.
Magnetic resonance imaging (MRI) may be helpful in screening women with cosmetic breast implants, but this technology is expensive, and evidence supporting its routine use in this population is limited.
Some mammographers use special techniques to better visualize the breast tissue of women with implants. These techniques include displacing the implant posteriorly and pulling the breast tissue in front of it. However, even with such strategies, as much as one-third of the breast tissue may be inadequately assessed.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These findings underscore the importance of sharing the risks of nonlocalized breast malignancy and increased breast cancer mortality with patients who are considering cosmetic breast implants, as well as with women who have already undergone this common procedure. Future studies are needed to address relevant issues, including the role of 3-D (tomosynthesis) technology in screening women with breast implants and optimal screening intervals in this subgroup.
ANDREW M. KAUNITZ, MD
We want to hear from you. Tell us what you think.
Long-term increase seen in abdominal sacrocolpopexy failure rates
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.
Should have used other dystocia maneuvers first
gb
AN OBGYN ENCOUNTERED SHOULDER DYSTOCIA. He used fundal pressure and downward lateral traction to free the baby’s shoulder. The child has a brachial plexus injury of the right shoulder, including nerve avulsion, a fractured clavicle, and permanent disfigurement. She underwent surgery; physical and occupational therapy will continue.
PARENTS' CLAIM The standard sequence of maneuvers should have been attempted before fundal pressure and lateral traction were used—the baby was sufficiently oxygenated to allow time for these maneuvers. Excessive lateral traction caused the injury.
DEFENDANTS' DEFENSE The injuries occurred in utero before or while the fetus progressed down the birth canal, and were due to the maternal forces of labor.
VERDICT A $3,070,000 Michigan verdict was returned against the hospital, ObGyn, and ObGyn group.
WHAT IS THE STANDARD SEQUENCE OF MANEUVERS FOR SHOULDER DYSTOCIA?
Read Dr. Robert L. Barbieri’s May Editorial, You are the second responder to a shoulder dystocia emergency. What do you do first? and Dr. Ronald T. Burkman’s March Stop/Start article, Stop all activities that may lead to further shoulder impaction when you suspect possible shoulder dystocia Meconium aspiration leads to brain injury
LATE IN HER PREGNANCY, a woman went to the emergency department (ED) with hypertension; she was discharged the same day. She saw her ObGyns, Dr. A and Dr. B, three times in the next 2 weeks. A day after her last visit, she returned to the ED in active labor. Dr. B assumed her care. Fetal monitoring indicated a nonreassuring heart rate with decelerations. Dr. B administered oxytocin and labor continued.
The baby was born by cesarean delivery after 25 minutes of fetal bradycardia. She was covered in meconium, with a low heart rate and irregular, labored respirations. The baby was transferred to another hospital, where she was treated for pulmonary hypertension, meconium aspiration, and seizures. The child is totally disabled, and will require constant care for life.
PARENTS' CLAIM The mother’s hypertension was not properly treated. Dr. B and the nurse waited too long to perform a cesarean delivery.
DEFENDANTS' DEFENSE Proper prenatal care was provided. There was no reason for additional testing; fetal heart tones at the mother’s last office visit were reactive. There were no clinical signs of a hematoma or cord varix during office visits. An unpredictable, unpreventable umbilical cord hematoma caused ischemia and hypoxia, and the subsequent brain injury. Meconium had been in the amniotic fluid for at least 10 hours due to the ischemic/hypoxic episode. The hematoma formed between her last office visit and when the mother came to the hospital the next day.
VERDICT Settlements were reached with Dr. A and the hospital. An Arkansas defense verdict was returned for Dr. B and the nurse.
14 months' recovery after mass removed
A GYNECOLOGIC ONCOLOGIST operated on a woman in her 50s to remove a large, noncancerous pelvic mass. The patient, discharged on postoperative day 2, was readmitted the next day with a fever (temperature, 103ºF), nausea, vomiting, and abdominal pain. Four days later, the oncologist repaired a perforated bowel and created an ileostomy. Other procedures were needed to drain abscesses and repair fistulas, and resect a large portion of colon due to continuing infection. Treatment lasted 14 months.
PATIENT'S CLAIM The gynecologic oncologist was negligent in failing to timely diagnose and treat the bowel perforation. Earlier repair would have curtailed development of the abscesses and fistulae.
PHYSICIAN'S DEFENSE Any complications the patient experienced were unrelated to any delay in treatment.
VERDICT A $612,237 Michigan verdict was returned.
Colon perforated during abdominal access
WHEN A MORBIDLY OBESE 37-YEAR-OLD WOMAN reported chronic pelvic pain, her gynecologist suspected endometriosis. Conservative treatment failed and the gynecologist offered laparoscopic hysterectomy.
After abdominal insufflation was unsuccessfully attempted twice using a Veress needle, the gynecologist entered the abdomen with a Visiport optical trocar, and continued the procedure. The gynecologist inspected the abdomen before closing but found no injuries.
The patient did not do well after surgery. CT scan detected a bowel perforation on postoperative day 6. During exploratory laparotomy, a through-and-through “bayonet” colon perforation was repaired. Because of the extensive infection, the patient’s surgical wound was left open and several “washouts” were performed; the wound was closed several weeks later. The patient also underwent two adhesiolysis procedures.
PATIENT'S CLAIM Access to the abdomen was not properly performed and caused colon perforation. The injury should have been found and treated earlier.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT A $750,000 Virginia settlement was reached.
READ How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy, by Michael Baggish, MD (Surgical Techniques, October 2012) What caused this C. diff infection after hysterectomy?
AFTER A HYSTERECTOMY, a 42-year-old woman developed a persistent fever and increased white blood cell count. The gynecologist prescribed ciprofloxacin for a urinary tract infection, and discharged the patient from the hospital on postoperative day 4. She returned to the gynecologist’s office with severe abdominal pain and vomiting 4 days after discharge. The gynecologist prescribed an antacid and told her to continue taking ciprofloxacin.
The patient was taken to the ED by ambulance 3 days later. Testing revealed a Clostridium dificule (C. diff) infection. During emergency surgery, a large portion of her colon was resected, and a colostomy was performed. The colostomy was reversed 6 months later. The patient developed an incisional hernia and has abdominal scarring.
PATIENT'S CLAIM Prophylactic antibiotics should have been prescribed before surgery.
Two possible scenarios were presented: 1) A bowel injury occurred during surgery, and ciprofloxacin likely worsened the infection caused by the bowel injury; or 2) ciprofloxacin triggered the C. diff infection that caused leaking colon perforations and subsequent peritonitis.
The colon perforations could have been avoided if the gynecologist had diagnosed and treated the C. diff infection in a timely manner.
PHYSICIAN'S DEFENSE The patient’s symptoms did not suggest a C. diff infection; testing was not necessary. Ciprofloxacin might have allowed the proliferation of the C. diff infection, but the use of the drug was not negligent. The infection was not preventable and could not have been diagnosed earlier.
VERDICT A $776,000 New York verdict was returned.
Brain injury and cerebral palsy: When did this occur?
DURING LABOR AND DELIVERY, there were periods when the fetal heart-rate tracings were nonreassuring with variable decelerations and fetal tachycardia; some variables were severe. The child suffered anoxic encephalopathy that caused neurologic injury and cerebral palsy.
PARENTS' CLAIM The infant suffered numerous hypoxic incidents before cesarean delivery was performed. An earlier cesarean delivery could have prevented the injury.
PHYSICIAN'S DEFENSE The newborn had a normal blood cord gas level of 7.2 pH and Apgar scores of 9 and 10, at 1 and 5 minutes, respectively. Fetal heart-rate tracings did not show evidence of fetal hypoxia. The brain injury likely occurred prior to the onset of labor and was possibly related to a viral encephalopathy.
VERDICT A Virginia defense verdict was returned. These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
gb
AN OBGYN ENCOUNTERED SHOULDER DYSTOCIA. He used fundal pressure and downward lateral traction to free the baby’s shoulder. The child has a brachial plexus injury of the right shoulder, including nerve avulsion, a fractured clavicle, and permanent disfigurement. She underwent surgery; physical and occupational therapy will continue.
PARENTS' CLAIM The standard sequence of maneuvers should have been attempted before fundal pressure and lateral traction were used—the baby was sufficiently oxygenated to allow time for these maneuvers. Excessive lateral traction caused the injury.
DEFENDANTS' DEFENSE The injuries occurred in utero before or while the fetus progressed down the birth canal, and were due to the maternal forces of labor.
VERDICT A $3,070,000 Michigan verdict was returned against the hospital, ObGyn, and ObGyn group.
WHAT IS THE STANDARD SEQUENCE OF MANEUVERS FOR SHOULDER DYSTOCIA?
Read Dr. Robert L. Barbieri’s May Editorial, You are the second responder to a shoulder dystocia emergency. What do you do first? and Dr. Ronald T. Burkman’s March Stop/Start article, Stop all activities that may lead to further shoulder impaction when you suspect possible shoulder dystocia Meconium aspiration leads to brain injury
LATE IN HER PREGNANCY, a woman went to the emergency department (ED) with hypertension; she was discharged the same day. She saw her ObGyns, Dr. A and Dr. B, three times in the next 2 weeks. A day after her last visit, she returned to the ED in active labor. Dr. B assumed her care. Fetal monitoring indicated a nonreassuring heart rate with decelerations. Dr. B administered oxytocin and labor continued.
The baby was born by cesarean delivery after 25 minutes of fetal bradycardia. She was covered in meconium, with a low heart rate and irregular, labored respirations. The baby was transferred to another hospital, where she was treated for pulmonary hypertension, meconium aspiration, and seizures. The child is totally disabled, and will require constant care for life.
PARENTS' CLAIM The mother’s hypertension was not properly treated. Dr. B and the nurse waited too long to perform a cesarean delivery.
DEFENDANTS' DEFENSE Proper prenatal care was provided. There was no reason for additional testing; fetal heart tones at the mother’s last office visit were reactive. There were no clinical signs of a hematoma or cord varix during office visits. An unpredictable, unpreventable umbilical cord hematoma caused ischemia and hypoxia, and the subsequent brain injury. Meconium had been in the amniotic fluid for at least 10 hours due to the ischemic/hypoxic episode. The hematoma formed between her last office visit and when the mother came to the hospital the next day.
VERDICT Settlements were reached with Dr. A and the hospital. An Arkansas defense verdict was returned for Dr. B and the nurse.
14 months' recovery after mass removed
A GYNECOLOGIC ONCOLOGIST operated on a woman in her 50s to remove a large, noncancerous pelvic mass. The patient, discharged on postoperative day 2, was readmitted the next day with a fever (temperature, 103ºF), nausea, vomiting, and abdominal pain. Four days later, the oncologist repaired a perforated bowel and created an ileostomy. Other procedures were needed to drain abscesses and repair fistulas, and resect a large portion of colon due to continuing infection. Treatment lasted 14 months.
PATIENT'S CLAIM The gynecologic oncologist was negligent in failing to timely diagnose and treat the bowel perforation. Earlier repair would have curtailed development of the abscesses and fistulae.
PHYSICIAN'S DEFENSE Any complications the patient experienced were unrelated to any delay in treatment.
VERDICT A $612,237 Michigan verdict was returned.
Colon perforated during abdominal access
WHEN A MORBIDLY OBESE 37-YEAR-OLD WOMAN reported chronic pelvic pain, her gynecologist suspected endometriosis. Conservative treatment failed and the gynecologist offered laparoscopic hysterectomy.
After abdominal insufflation was unsuccessfully attempted twice using a Veress needle, the gynecologist entered the abdomen with a Visiport optical trocar, and continued the procedure. The gynecologist inspected the abdomen before closing but found no injuries.
The patient did not do well after surgery. CT scan detected a bowel perforation on postoperative day 6. During exploratory laparotomy, a through-and-through “bayonet” colon perforation was repaired. Because of the extensive infection, the patient’s surgical wound was left open and several “washouts” were performed; the wound was closed several weeks later. The patient also underwent two adhesiolysis procedures.
PATIENT'S CLAIM Access to the abdomen was not properly performed and caused colon perforation. The injury should have been found and treated earlier.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT A $750,000 Virginia settlement was reached.
READ How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy, by Michael Baggish, MD (Surgical Techniques, October 2012) What caused this C. diff infection after hysterectomy?
AFTER A HYSTERECTOMY, a 42-year-old woman developed a persistent fever and increased white blood cell count. The gynecologist prescribed ciprofloxacin for a urinary tract infection, and discharged the patient from the hospital on postoperative day 4. She returned to the gynecologist’s office with severe abdominal pain and vomiting 4 days after discharge. The gynecologist prescribed an antacid and told her to continue taking ciprofloxacin.
The patient was taken to the ED by ambulance 3 days later. Testing revealed a Clostridium dificule (C. diff) infection. During emergency surgery, a large portion of her colon was resected, and a colostomy was performed. The colostomy was reversed 6 months later. The patient developed an incisional hernia and has abdominal scarring.
PATIENT'S CLAIM Prophylactic antibiotics should have been prescribed before surgery.
Two possible scenarios were presented: 1) A bowel injury occurred during surgery, and ciprofloxacin likely worsened the infection caused by the bowel injury; or 2) ciprofloxacin triggered the C. diff infection that caused leaking colon perforations and subsequent peritonitis.
The colon perforations could have been avoided if the gynecologist had diagnosed and treated the C. diff infection in a timely manner.
PHYSICIAN'S DEFENSE The patient’s symptoms did not suggest a C. diff infection; testing was not necessary. Ciprofloxacin might have allowed the proliferation of the C. diff infection, but the use of the drug was not negligent. The infection was not preventable and could not have been diagnosed earlier.
VERDICT A $776,000 New York verdict was returned.
Brain injury and cerebral palsy: When did this occur?
DURING LABOR AND DELIVERY, there were periods when the fetal heart-rate tracings were nonreassuring with variable decelerations and fetal tachycardia; some variables were severe. The child suffered anoxic encephalopathy that caused neurologic injury and cerebral palsy.
PARENTS' CLAIM The infant suffered numerous hypoxic incidents before cesarean delivery was performed. An earlier cesarean delivery could have prevented the injury.
PHYSICIAN'S DEFENSE The newborn had a normal blood cord gas level of 7.2 pH and Apgar scores of 9 and 10, at 1 and 5 minutes, respectively. Fetal heart-rate tracings did not show evidence of fetal hypoxia. The brain injury likely occurred prior to the onset of labor and was possibly related to a viral encephalopathy.
VERDICT A Virginia defense verdict was returned. These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
gb
AN OBGYN ENCOUNTERED SHOULDER DYSTOCIA. He used fundal pressure and downward lateral traction to free the baby’s shoulder. The child has a brachial plexus injury of the right shoulder, including nerve avulsion, a fractured clavicle, and permanent disfigurement. She underwent surgery; physical and occupational therapy will continue.
PARENTS' CLAIM The standard sequence of maneuvers should have been attempted before fundal pressure and lateral traction were used—the baby was sufficiently oxygenated to allow time for these maneuvers. Excessive lateral traction caused the injury.
DEFENDANTS' DEFENSE The injuries occurred in utero before or while the fetus progressed down the birth canal, and were due to the maternal forces of labor.
VERDICT A $3,070,000 Michigan verdict was returned against the hospital, ObGyn, and ObGyn group.
WHAT IS THE STANDARD SEQUENCE OF MANEUVERS FOR SHOULDER DYSTOCIA?
Read Dr. Robert L. Barbieri’s May Editorial, You are the second responder to a shoulder dystocia emergency. What do you do first? and Dr. Ronald T. Burkman’s March Stop/Start article, Stop all activities that may lead to further shoulder impaction when you suspect possible shoulder dystocia Meconium aspiration leads to brain injury
LATE IN HER PREGNANCY, a woman went to the emergency department (ED) with hypertension; she was discharged the same day. She saw her ObGyns, Dr. A and Dr. B, three times in the next 2 weeks. A day after her last visit, she returned to the ED in active labor. Dr. B assumed her care. Fetal monitoring indicated a nonreassuring heart rate with decelerations. Dr. B administered oxytocin and labor continued.
The baby was born by cesarean delivery after 25 minutes of fetal bradycardia. She was covered in meconium, with a low heart rate and irregular, labored respirations. The baby was transferred to another hospital, where she was treated for pulmonary hypertension, meconium aspiration, and seizures. The child is totally disabled, and will require constant care for life.
PARENTS' CLAIM The mother’s hypertension was not properly treated. Dr. B and the nurse waited too long to perform a cesarean delivery.
DEFENDANTS' DEFENSE Proper prenatal care was provided. There was no reason for additional testing; fetal heart tones at the mother’s last office visit were reactive. There were no clinical signs of a hematoma or cord varix during office visits. An unpredictable, unpreventable umbilical cord hematoma caused ischemia and hypoxia, and the subsequent brain injury. Meconium had been in the amniotic fluid for at least 10 hours due to the ischemic/hypoxic episode. The hematoma formed between her last office visit and when the mother came to the hospital the next day.
VERDICT Settlements were reached with Dr. A and the hospital. An Arkansas defense verdict was returned for Dr. B and the nurse.
14 months' recovery after mass removed
A GYNECOLOGIC ONCOLOGIST operated on a woman in her 50s to remove a large, noncancerous pelvic mass. The patient, discharged on postoperative day 2, was readmitted the next day with a fever (temperature, 103ºF), nausea, vomiting, and abdominal pain. Four days later, the oncologist repaired a perforated bowel and created an ileostomy. Other procedures were needed to drain abscesses and repair fistulas, and resect a large portion of colon due to continuing infection. Treatment lasted 14 months.
PATIENT'S CLAIM The gynecologic oncologist was negligent in failing to timely diagnose and treat the bowel perforation. Earlier repair would have curtailed development of the abscesses and fistulae.
PHYSICIAN'S DEFENSE Any complications the patient experienced were unrelated to any delay in treatment.
VERDICT A $612,237 Michigan verdict was returned.
Colon perforated during abdominal access
WHEN A MORBIDLY OBESE 37-YEAR-OLD WOMAN reported chronic pelvic pain, her gynecologist suspected endometriosis. Conservative treatment failed and the gynecologist offered laparoscopic hysterectomy.
After abdominal insufflation was unsuccessfully attempted twice using a Veress needle, the gynecologist entered the abdomen with a Visiport optical trocar, and continued the procedure. The gynecologist inspected the abdomen before closing but found no injuries.
The patient did not do well after surgery. CT scan detected a bowel perforation on postoperative day 6. During exploratory laparotomy, a through-and-through “bayonet” colon perforation was repaired. Because of the extensive infection, the patient’s surgical wound was left open and several “washouts” were performed; the wound was closed several weeks later. The patient also underwent two adhesiolysis procedures.
PATIENT'S CLAIM Access to the abdomen was not properly performed and caused colon perforation. The injury should have been found and treated earlier.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT A $750,000 Virginia settlement was reached.
READ How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy, by Michael Baggish, MD (Surgical Techniques, October 2012) What caused this C. diff infection after hysterectomy?
AFTER A HYSTERECTOMY, a 42-year-old woman developed a persistent fever and increased white blood cell count. The gynecologist prescribed ciprofloxacin for a urinary tract infection, and discharged the patient from the hospital on postoperative day 4. She returned to the gynecologist’s office with severe abdominal pain and vomiting 4 days after discharge. The gynecologist prescribed an antacid and told her to continue taking ciprofloxacin.
The patient was taken to the ED by ambulance 3 days later. Testing revealed a Clostridium dificule (C. diff) infection. During emergency surgery, a large portion of her colon was resected, and a colostomy was performed. The colostomy was reversed 6 months later. The patient developed an incisional hernia and has abdominal scarring.
PATIENT'S CLAIM Prophylactic antibiotics should have been prescribed before surgery.
Two possible scenarios were presented: 1) A bowel injury occurred during surgery, and ciprofloxacin likely worsened the infection caused by the bowel injury; or 2) ciprofloxacin triggered the C. diff infection that caused leaking colon perforations and subsequent peritonitis.
The colon perforations could have been avoided if the gynecologist had diagnosed and treated the C. diff infection in a timely manner.
PHYSICIAN'S DEFENSE The patient’s symptoms did not suggest a C. diff infection; testing was not necessary. Ciprofloxacin might have allowed the proliferation of the C. diff infection, but the use of the drug was not negligent. The infection was not preventable and could not have been diagnosed earlier.
VERDICT A $776,000 New York verdict was returned.
Brain injury and cerebral palsy: When did this occur?
DURING LABOR AND DELIVERY, there were periods when the fetal heart-rate tracings were nonreassuring with variable decelerations and fetal tachycardia; some variables were severe. The child suffered anoxic encephalopathy that caused neurologic injury and cerebral palsy.
PARENTS' CLAIM The infant suffered numerous hypoxic incidents before cesarean delivery was performed. An earlier cesarean delivery could have prevented the injury.
PHYSICIAN'S DEFENSE The newborn had a normal blood cord gas level of 7.2 pH and Apgar scores of 9 and 10, at 1 and 5 minutes, respectively. Fetal heart-rate tracings did not show evidence of fetal hypoxia. The brain injury likely occurred prior to the onset of labor and was possibly related to a viral encephalopathy.
VERDICT A Virginia defense verdict was returned. These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
Does myo-inositol supplementation reduce the rate of gestational diabetes in pregnant women with a family history of type 2 diabetes?
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.