User login
A 67-year-old woman with bilateral hand numbness
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
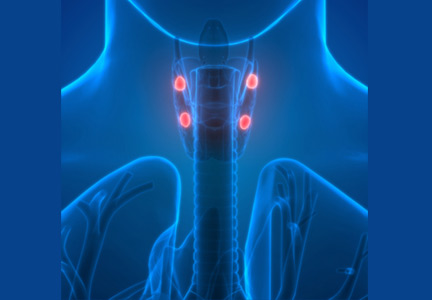
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
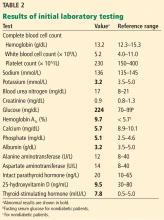
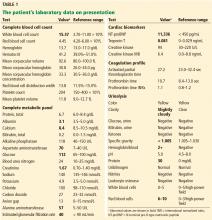
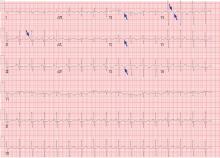
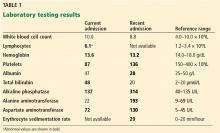
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.
A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
A 50-year-old woman with new-onset seizure
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
Big heart, small ring
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.
Fever after recent travel
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
Abdominal pain and bloody diarrhea in a 32-year-old woman
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.