User login
Cracking the clinician educator code in gastroenterology
For gastroenterologists who enter academic medicine, the most common career track is that of clinician educator (CE). Although most academic gastroenterologists are CEs, their career paths vary substantially, and expectations for promotion can be much less explicit compared with those of physician scientists. This delineation of different pathways in academic gastroenterology starts as early as the fellowship application process, before the implications are understood. Furthermore, many community gastroenterologists have appointments within academic medical centers, which typically fall into the realm of CEs.
A review of all gastroenterology and hepatology fellowship program websites listed on the American Gastroenterological Association website showed that 33 of 175 (18.8%) programs endorse distinctly different tracks, usually distinguishing traditional research (i.e., basic science, epidemiology, or outcomes) from clinical care of patients (i.e., clinician educator or clinical scholar). One of the most common words appearing in descriptions of both tracks was “clinical,” highlighting that a good clinician educator or researcher is, first and foremost, a good clinician.
With clinical duties requiring the majority of a CE’s time and efforts, a reasonable assumption is that CEs are clinicians who teach trainees via lectures, clinic, endoscopy, and/or inpatient rounds. Although such educational endeavors form the backbone of a CE’s scholarly activities, what constitutes scholarship for CEs is much more diverse than many people realize. The variability in what a career as a CE may look like can be both an obstacle and an opportunity. Included in the category of CE are community clinicians who have a stake in the education of residents and fellows and play an important role in trainee learning. Sherbino et al1 defined a CE as “a clinician active in health professional practice who applies theory to education practice, engages in education scholarship, and serves as a consultant to other health professionals on education issues.”
Because we recognize that many community and academic gastroenterologists spend the majority of their education efforts teaching trainees, we have made every effort to ensure that the five recommendations listed later are equally pertinent to all gastroenterologists who devote any portion of their careers to educating trainees, colleagues, allied health professionals, as well as patients. For example, a CE who primarily teaches trainees still can benefit from learning how to better document their efforts, receive mentorship as an educator, take everyday activities and convert them into scholarship, share teaching materials with broader audiences, and learn new teaching techniques without ever opening a book on education theory. For community-based physicians, this can assist in obtaining recognition from the academic centers for their teaching efforts. We hope that the five recommendations that follow will serve to guide those just setting out on the CE path, as well as for those who have trodden it for some time.
Number 1: Maintain a current curriculum vitae and teaching portfolio
All CEs must have two critical instruments to document their accomplishments to their institutions and to the field: a curriculum vitae (CV) and a teaching portfolio. These items also are very important when the time comes for promotion because they validate one’s accomplishments, both quantitatively and qualitatively. Knowing the criteria for promotion as a CE is critical for shaping one’s career, and we recommend checking with an individual’s institution for its specific requirements regarding formats for both the CV and the teaching portfolio, which typically are available from the academic promotion committee. Because most fellows and faculty are familiar with the format of a CV, we will focus on the teaching portfolio.
For most fellows and many faculty, the teaching portfolio is a new and/or less well understood entity. Unlike a CV, the teaching portfolio presents teaching activities not only as a collection or list, but also provides evidence of the influence the work has had on others, in a much more personal way. A few tips are listed on putting together a teaching portfolio. However, the most important advice we can offer is this: one should save all evidence of teaching including unsolicited letters and e-mails from learners and colleagues.
If your institution does not have a teaching portfolio template, we recommend using a pre-existing format. Several examples from academic medicine can be found on the Internet or on MedEdPORTAL, an open-access repository of educational content provided by the Association of American Medical Colleges. One such tool is the Educator Portfolio Template of the Academic Pediatric Association’s Educational Scholars Program (available: https://www.academicpeds.org/education/educator_portfolio_template.cfm). The Association of American Medical Colleges Group on Education Affairs held a consensus conference in 2006, from which five educational categories were defined: teaching, learner assessment, curriculum development, mentoring and advising, and educational leadership and administration.2 These categories can serve as an arrangement for a teaching portfolio. We also recommend that you include both educational research/scholarship and web-based educational materials such as online learning modules, YouTube videos, blogs, and wikis as a part of a teaching portfolio. For each project highlighted in the teaching portfolio, we recommend reflecting on and writing down how the project shows the quantity and quality of the work.
Quantity of work in the teaching portfolio refers to more than a mere cataloging of published peer-reviewed articles and book chapters, courses taught, presentations given, and so forth (which should be included in the CV). Instead, it documents time spent in teaching activities, how often teaching occurs, the number and types of learners involved, and how the activity fits into a training program.
Quality of work can include how innovative methods were crafted and implemented to customize teaching in creative ways to accomplish specific learning objectives. This description renders the contents of the teaching portfolio more than merely a sketch of work activities documented by numbers, and tells a story about what occurred. When documenting evidence of quality, provide comparative measures whenever possible. Quality of teaching also can be illustrated by evaluations, pretests and posttests, and as complimentary e-mails and letters from learners and other faculty members. The description of teaching activities also shows one’s flexibility as an educator, and the greater the breadth of experiences, the better. A CE also must document within the portfolio how the teaching activity drew from existing literature and best practices and/or contributed to the medical education field and its body of knowledge. Above all else, we recommend collecting evidence on teaching to both provide evidence of one’s teaching skills and to gather data on which to improve.
The teaching portfolio templates begin with a personal statement outlining why one teaches (i.e., teaching philosophy). In a teaching portfolio, it is important to include details of how impact was defined or determined with regard to teaching endeavors, how the feedback from formal evaluative processes was used to mold one’s future activities as an educator, and what strategies will be implemented to improve teaching to meet the needs of diverse and changing groups of learners.
Both the CV and teaching portfolio should be updated continually – we recommend at least quarterly (or as articles are published, courses are taught, abstracts are presented, and so forth) – to ensure that nothing is overlooked or forgotten.
Number 2: Mentors and mentees
Every CE needs to have a primary mentor, typically a more senior faculty member with an interest in and experience with mentoring, as well as a commitment to fostering the mentee’s professional growth. It may be difficult to find a mentor when starting out as a junior faculty member or when changing academic institutions. Once you have a mentor, take ownership for the success of the relationship by managing-up, by organizing all the meetings, exceeding (not just meeting) deadlines, and by communicating needs and information in a way the mentor prefers. Rustgi and Hecht3 wrap up their article on mentorship with a pathway that highlights the following components for a successful mentoring relationship: regular meetings, specific goals and measurable outcomes, manuscript and grant writing, presentation skills and efficiency, and navigating the complexities of regulatory affairs such as institutional review boards. Although many of these tenets hold true for both clinician researchers and clinician educators, Farrell et al4 offer four steps to finding a mentor for clinician educators, as follows. Step 1: self-reflection and assessment: critically assessing one’s competence as a teacher, educational administrator, or researcher; determining what prior education projects have been successful and why; and defining career goals and the current relationship to them. Step 2: identification of areas needing development: examples may include teaching skills, curriculum innovation, evaluation/assessment, educational research, time management, negotiation skills, grantsmanship, scholarly writing, and presentation skills; identify specific questions regarding the type of help needed. Step 3: matchmaking: determine qualities (personal and professional) desired in a mentor, and search for candidates with the help of colleagues. Step 4: engagement with a mentor: explain why you desire mentorship, career goals, current academic role(s), your perceived needs, and recognize and acknowledge appreciation for your mentor’s time and energy.
One caution is to avoid having too many primary mentors. A mentee may assume the perspective that it takes a village when it comes to seeking and providing mentoring. Although having clinical, research, and/or personal mentors can be helpful, having too many mentors can make it difficult to meet regularly enough to allow for the mentee–mentor relationship to grow. Instead of a network of mentors, build a web of minimentors, or coaches, to serve as consultants, coaches, and accountability partners, and tap into this network as needed. Mentors are involved longitudinally with mentees and tend to provide general career and project-specific guidance, whereas coaches tend to be involved in specific projects or areas of focus of a mentee.
In addition to having their own mentors, CEs quickly will find opportunities themselves to serve as mentors to more junior faculty, fellows, residents, and students. Indeed, one measure of a successful mentor–mentee relationship is the development of the mentee into a new mentor for future generations.
Number 3: Think broadly about scholarship
Traditionally, the definition of scholarship has been very narrow and usually is related to the number of publications and grants one receives. Beginning with Boyer’s work in 1990, the definition of scholarship has expanded at academic institutions beyond the concept of traditional research.5 Medical education scholarship most often is guided and judged by six core qualitative standards of excellence, known as “Glassick’s criteria”6: clear goals, adequate preparation, appropriate methods, significant results, effective presentation, and reflective critique. The key to scholarship is that it builds on or adds to the field, is made public, and thus available for peer-review.
CE projects can be categorized in many ways, but we recommend broadening the classic notions of research with which we have been indoctrinated. Golub’s7 2016 editorial in the Journal of the American Medical Association, “Looking Inward and Reflecting Back: Medical Education and Journal of the American Medical Association,” highlights the range of research questions and methodologies, which include ethics, behavioral psychology, diversity of patient care and the workforce, medical education research, quality and value of care, well-being of trainees and faculty, and health informatics. If one breaks down daily tasks, countless opportunities for scholarly projects will emerge. One need look no further for opportunities than the countless opportunities for quality improvement research that avail themselves daily, with examples ranging from reducing variation in cirrhosis care to improving adenoma detection rates. Quality improvement is an important method of scholarship for both academic and community-based physicians, which also can contribute toward Part IV of Maintenance of Certification requirements. CEs also can engage in educational scholarship other than research by using these same principles. To transform your teaching into scholarship you should examine the activities you perform or a problem that needs to be solved, apply information or a solution based on best practices or what is known from the literature, and then share the results/products with others (peer-review). Crites et al8 provide practical guidelines for developing education research questions, designing and implementing scholarly activities, and interpreting the scope and impact of education scholarship.
In addition, reaching beyond one’s department to other departments, as well as participating in educational scholarly activities on regional and national levels, is important as one’s career progresses. Well-connected and diverse networks are information highways by which one’s work can be amplified to achieve a greater impact, and from which many opportunities will be shared.
Number 4: Share broadly
Scholarship activities of both academic and community-based CEs can target many audiences, including medical students, residents, and fellows; faculty; other health professions; or even patients and the community. Knowing who will be the recipients or end-users can help to identify which types of projects may be most rewarding and make the greatest impact. Consider sharing curricula, evaluation tools, and other educational products with colleagues at other institutions who ask for them. Request acknowledgment for the development of the materials and ask for written feedback on how these products are being used and what impact they have had on learners.
One education model used to assess the impact and target of education interventions is known as Kirkpatrick’s9 hierarchy, which traditionally included the following four levels: reaction (level 1), learning (level 2), behavior (level 3), and results (level 4). The model has been adapted by the British Medical Journal’s Best Evidence in Medical Education collaboration to medical education with the following modifications in levels as follows.9,10 Level 1: participation: focused on learners’ views of the learning experience including content, presentation, and teaching methods. Level 2a: modification of attitudes/perceptions: focused on changes in attitudes or perceptions between participant groups toward the intervention. Level 2b: modification of knowledge/skills: for knowledge, focused on the acquisition of concepts, procedures, and principles; for skills, focused on the acquisition of problem solving, psychomotor, and social skills. Level 3: behavioral change: focused on the transfer of learning to the workplace or willingness of learners to apply new knowledge and skills. Level 4a: change in organizational practice: focused on wider changes in the organization or delivery of care attributable to an educational program. Level 4b: focused on improvements in the health and well-being of patients as a direct result of an education initiative.
Similar to more traditional clinical research, education research needs to be performed in a scholarly fashion and shared with a wider audience. In addition to submitting research to gastroenterology journals (e.g., Gastroenterology’s Mentoring, Education, and Training Corner), education research can be submitted to education journals such as the Association of American Medical Colleges’ Academic Medicine, the Association for the Study of Medical Education’s Medical Education, the Accreditation Council for Graduate Medical Education’s Journal of Graduate Medical Education, or the European Association for Medical Education in Europe’s Medical Teacher; online education warehouses such as MedEdPORTAL (www.mededportal.org) or MERLOT (www.merlot.org); and national conferences as workshops. Also, one should keep in mind that opportunities arise on a regular basis to share educational videos or images in forums such as the American Society for Gastrointestinal Endoscopy’s video journal VideoGIE, The American Journal of Gastroenterology’s video of the month, and Clinical Gastroenterology and Hepatology’s Images of the Month.
Number 5: Ongoing professional development
Continuing Medical Education is a standard requirement to maintain an active medical license because it shows ongoing efforts to remain up-to-date with changes in medicine. Similar opportunities exist with respect to further development as an educator. Given the multitude of manners in which these opportunities can be divided, we have compiled recommendations for resources on educational scholarship based on level of experience and desired level of engagement (Table 1).
Summary
The framework provided should help guide the gastroenterologist on the path of becoming an effective clinician educator in gastroenterology. The diversity of what a career as a clinician educator can entail is unlimited. The success of the future of medical education and our careers requires not only that every clinician educator be productive, but also that each one brings a unique passion to work each day to share. The authors would like to thank all those clinician educators who contributed to our education, and look forward to learning from you in the future.
Acknowledgments
The authors thank Dr Lee Ligon, Center for Research, Innovation, and Scholarship, Department of Pediatrics, Baylor College of Medicine, for providing editorial assistance.
References
1. Sherbino, J., Frank, J.R., Snell, L. Defining the key roles and competencies of the clinician-educator of the 21st century: a national mixed-methods study. Acad Med. 2014;89:783-9.
2. Simpson, D., Fincher, R.M., Hafler, J.P., et al. Advancing educators and education by defining the components and evidence associated with educational scholarship. Med Educ. 2007;41:1002-9.
3. Rustgi, A.K. Hecht, G.A. Mentorship in academic medicine. Gastroenterology. 2011;141:789-92.
4. Farrell, S.E., Digioia, N.M., Broderick, K.B., et al. Mentoring for clinician-educators. Acad Emerg Med. 2004;11:1346-50.
5. Boyer, E.L. Scholarship reconsidered: priorities of the professoriate. Carnegie Foundation for the Advancement of Teaching, Princeton, NJ; 1990.
6. Glassick, C.E., Taylor-Huber, M., Maeroff, G.I., et al. Scholarship assessed: evaluation of the professoriate. Jossey-Bass, San Francisco; 1997.
7. Golub, R.M. Looking inward and reflecting back medical education and JAMA. JAMA. 2016;316:2200-3.
8. Crites, G.E., Gaines, J.K., Cottrell, S., et al. Medical education scholarship: an introductory guide: AMEE guide no. 89. Med Teach. 2014;36:657-74.
9. Kirkpatrick, D.L. Evaluation of training. In: R. Craig, L. Bittel (Eds.) Training and development handbook. McGraw-Hill, New York; 1967: 87-112.
10. Littlewood, S., Ypinazar, V., Margolis, S.A., et al. Early practical experience and the social responsiveness of clinical education: systematic review. BMJ. 2005;331:387-91.
Dr. Shapiro is a gastroenterology fellow in the department of medicine, section of gastroenterology; Dr. Gould Suarez is an associate professor in the department of medicine, section of gastroenterology and associate program director of the gastroenterology fellowship; and Dr. Turner is an associate professor of pediatrics, vice chair of education, associate program director for house staff education, section of academic general pediatrics, and director for research, innovation, and scholarship, Baylor College of Medicine, Houston. The authors disclose no conflicts.
For gastroenterologists who enter academic medicine, the most common career track is that of clinician educator (CE). Although most academic gastroenterologists are CEs, their career paths vary substantially, and expectations for promotion can be much less explicit compared with those of physician scientists. This delineation of different pathways in academic gastroenterology starts as early as the fellowship application process, before the implications are understood. Furthermore, many community gastroenterologists have appointments within academic medical centers, which typically fall into the realm of CEs.
A review of all gastroenterology and hepatology fellowship program websites listed on the American Gastroenterological Association website showed that 33 of 175 (18.8%) programs endorse distinctly different tracks, usually distinguishing traditional research (i.e., basic science, epidemiology, or outcomes) from clinical care of patients (i.e., clinician educator or clinical scholar). One of the most common words appearing in descriptions of both tracks was “clinical,” highlighting that a good clinician educator or researcher is, first and foremost, a good clinician.
With clinical duties requiring the majority of a CE’s time and efforts, a reasonable assumption is that CEs are clinicians who teach trainees via lectures, clinic, endoscopy, and/or inpatient rounds. Although such educational endeavors form the backbone of a CE’s scholarly activities, what constitutes scholarship for CEs is much more diverse than many people realize. The variability in what a career as a CE may look like can be both an obstacle and an opportunity. Included in the category of CE are community clinicians who have a stake in the education of residents and fellows and play an important role in trainee learning. Sherbino et al1 defined a CE as “a clinician active in health professional practice who applies theory to education practice, engages in education scholarship, and serves as a consultant to other health professionals on education issues.”
Because we recognize that many community and academic gastroenterologists spend the majority of their education efforts teaching trainees, we have made every effort to ensure that the five recommendations listed later are equally pertinent to all gastroenterologists who devote any portion of their careers to educating trainees, colleagues, allied health professionals, as well as patients. For example, a CE who primarily teaches trainees still can benefit from learning how to better document their efforts, receive mentorship as an educator, take everyday activities and convert them into scholarship, share teaching materials with broader audiences, and learn new teaching techniques without ever opening a book on education theory. For community-based physicians, this can assist in obtaining recognition from the academic centers for their teaching efforts. We hope that the five recommendations that follow will serve to guide those just setting out on the CE path, as well as for those who have trodden it for some time.
Number 1: Maintain a current curriculum vitae and teaching portfolio
All CEs must have two critical instruments to document their accomplishments to their institutions and to the field: a curriculum vitae (CV) and a teaching portfolio. These items also are very important when the time comes for promotion because they validate one’s accomplishments, both quantitatively and qualitatively. Knowing the criteria for promotion as a CE is critical for shaping one’s career, and we recommend checking with an individual’s institution for its specific requirements regarding formats for both the CV and the teaching portfolio, which typically are available from the academic promotion committee. Because most fellows and faculty are familiar with the format of a CV, we will focus on the teaching portfolio.
For most fellows and many faculty, the teaching portfolio is a new and/or less well understood entity. Unlike a CV, the teaching portfolio presents teaching activities not only as a collection or list, but also provides evidence of the influence the work has had on others, in a much more personal way. A few tips are listed on putting together a teaching portfolio. However, the most important advice we can offer is this: one should save all evidence of teaching including unsolicited letters and e-mails from learners and colleagues.
If your institution does not have a teaching portfolio template, we recommend using a pre-existing format. Several examples from academic medicine can be found on the Internet or on MedEdPORTAL, an open-access repository of educational content provided by the Association of American Medical Colleges. One such tool is the Educator Portfolio Template of the Academic Pediatric Association’s Educational Scholars Program (available: https://www.academicpeds.org/education/educator_portfolio_template.cfm). The Association of American Medical Colleges Group on Education Affairs held a consensus conference in 2006, from which five educational categories were defined: teaching, learner assessment, curriculum development, mentoring and advising, and educational leadership and administration.2 These categories can serve as an arrangement for a teaching portfolio. We also recommend that you include both educational research/scholarship and web-based educational materials such as online learning modules, YouTube videos, blogs, and wikis as a part of a teaching portfolio. For each project highlighted in the teaching portfolio, we recommend reflecting on and writing down how the project shows the quantity and quality of the work.
Quantity of work in the teaching portfolio refers to more than a mere cataloging of published peer-reviewed articles and book chapters, courses taught, presentations given, and so forth (which should be included in the CV). Instead, it documents time spent in teaching activities, how often teaching occurs, the number and types of learners involved, and how the activity fits into a training program.
Quality of work can include how innovative methods were crafted and implemented to customize teaching in creative ways to accomplish specific learning objectives. This description renders the contents of the teaching portfolio more than merely a sketch of work activities documented by numbers, and tells a story about what occurred. When documenting evidence of quality, provide comparative measures whenever possible. Quality of teaching also can be illustrated by evaluations, pretests and posttests, and as complimentary e-mails and letters from learners and other faculty members. The description of teaching activities also shows one’s flexibility as an educator, and the greater the breadth of experiences, the better. A CE also must document within the portfolio how the teaching activity drew from existing literature and best practices and/or contributed to the medical education field and its body of knowledge. Above all else, we recommend collecting evidence on teaching to both provide evidence of one’s teaching skills and to gather data on which to improve.
The teaching portfolio templates begin with a personal statement outlining why one teaches (i.e., teaching philosophy). In a teaching portfolio, it is important to include details of how impact was defined or determined with regard to teaching endeavors, how the feedback from formal evaluative processes was used to mold one’s future activities as an educator, and what strategies will be implemented to improve teaching to meet the needs of diverse and changing groups of learners.
Both the CV and teaching portfolio should be updated continually – we recommend at least quarterly (or as articles are published, courses are taught, abstracts are presented, and so forth) – to ensure that nothing is overlooked or forgotten.
Number 2: Mentors and mentees
Every CE needs to have a primary mentor, typically a more senior faculty member with an interest in and experience with mentoring, as well as a commitment to fostering the mentee’s professional growth. It may be difficult to find a mentor when starting out as a junior faculty member or when changing academic institutions. Once you have a mentor, take ownership for the success of the relationship by managing-up, by organizing all the meetings, exceeding (not just meeting) deadlines, and by communicating needs and information in a way the mentor prefers. Rustgi and Hecht3 wrap up their article on mentorship with a pathway that highlights the following components for a successful mentoring relationship: regular meetings, specific goals and measurable outcomes, manuscript and grant writing, presentation skills and efficiency, and navigating the complexities of regulatory affairs such as institutional review boards. Although many of these tenets hold true for both clinician researchers and clinician educators, Farrell et al4 offer four steps to finding a mentor for clinician educators, as follows. Step 1: self-reflection and assessment: critically assessing one’s competence as a teacher, educational administrator, or researcher; determining what prior education projects have been successful and why; and defining career goals and the current relationship to them. Step 2: identification of areas needing development: examples may include teaching skills, curriculum innovation, evaluation/assessment, educational research, time management, negotiation skills, grantsmanship, scholarly writing, and presentation skills; identify specific questions regarding the type of help needed. Step 3: matchmaking: determine qualities (personal and professional) desired in a mentor, and search for candidates with the help of colleagues. Step 4: engagement with a mentor: explain why you desire mentorship, career goals, current academic role(s), your perceived needs, and recognize and acknowledge appreciation for your mentor’s time and energy.
One caution is to avoid having too many primary mentors. A mentee may assume the perspective that it takes a village when it comes to seeking and providing mentoring. Although having clinical, research, and/or personal mentors can be helpful, having too many mentors can make it difficult to meet regularly enough to allow for the mentee–mentor relationship to grow. Instead of a network of mentors, build a web of minimentors, or coaches, to serve as consultants, coaches, and accountability partners, and tap into this network as needed. Mentors are involved longitudinally with mentees and tend to provide general career and project-specific guidance, whereas coaches tend to be involved in specific projects or areas of focus of a mentee.
In addition to having their own mentors, CEs quickly will find opportunities themselves to serve as mentors to more junior faculty, fellows, residents, and students. Indeed, one measure of a successful mentor–mentee relationship is the development of the mentee into a new mentor for future generations.
Number 3: Think broadly about scholarship
Traditionally, the definition of scholarship has been very narrow and usually is related to the number of publications and grants one receives. Beginning with Boyer’s work in 1990, the definition of scholarship has expanded at academic institutions beyond the concept of traditional research.5 Medical education scholarship most often is guided and judged by six core qualitative standards of excellence, known as “Glassick’s criteria”6: clear goals, adequate preparation, appropriate methods, significant results, effective presentation, and reflective critique. The key to scholarship is that it builds on or adds to the field, is made public, and thus available for peer-review.
CE projects can be categorized in many ways, but we recommend broadening the classic notions of research with which we have been indoctrinated. Golub’s7 2016 editorial in the Journal of the American Medical Association, “Looking Inward and Reflecting Back: Medical Education and Journal of the American Medical Association,” highlights the range of research questions and methodologies, which include ethics, behavioral psychology, diversity of patient care and the workforce, medical education research, quality and value of care, well-being of trainees and faculty, and health informatics. If one breaks down daily tasks, countless opportunities for scholarly projects will emerge. One need look no further for opportunities than the countless opportunities for quality improvement research that avail themselves daily, with examples ranging from reducing variation in cirrhosis care to improving adenoma detection rates. Quality improvement is an important method of scholarship for both academic and community-based physicians, which also can contribute toward Part IV of Maintenance of Certification requirements. CEs also can engage in educational scholarship other than research by using these same principles. To transform your teaching into scholarship you should examine the activities you perform or a problem that needs to be solved, apply information or a solution based on best practices or what is known from the literature, and then share the results/products with others (peer-review). Crites et al8 provide practical guidelines for developing education research questions, designing and implementing scholarly activities, and interpreting the scope and impact of education scholarship.
In addition, reaching beyond one’s department to other departments, as well as participating in educational scholarly activities on regional and national levels, is important as one’s career progresses. Well-connected and diverse networks are information highways by which one’s work can be amplified to achieve a greater impact, and from which many opportunities will be shared.
Number 4: Share broadly
Scholarship activities of both academic and community-based CEs can target many audiences, including medical students, residents, and fellows; faculty; other health professions; or even patients and the community. Knowing who will be the recipients or end-users can help to identify which types of projects may be most rewarding and make the greatest impact. Consider sharing curricula, evaluation tools, and other educational products with colleagues at other institutions who ask for them. Request acknowledgment for the development of the materials and ask for written feedback on how these products are being used and what impact they have had on learners.
One education model used to assess the impact and target of education interventions is known as Kirkpatrick’s9 hierarchy, which traditionally included the following four levels: reaction (level 1), learning (level 2), behavior (level 3), and results (level 4). The model has been adapted by the British Medical Journal’s Best Evidence in Medical Education collaboration to medical education with the following modifications in levels as follows.9,10 Level 1: participation: focused on learners’ views of the learning experience including content, presentation, and teaching methods. Level 2a: modification of attitudes/perceptions: focused on changes in attitudes or perceptions between participant groups toward the intervention. Level 2b: modification of knowledge/skills: for knowledge, focused on the acquisition of concepts, procedures, and principles; for skills, focused on the acquisition of problem solving, psychomotor, and social skills. Level 3: behavioral change: focused on the transfer of learning to the workplace or willingness of learners to apply new knowledge and skills. Level 4a: change in organizational practice: focused on wider changes in the organization or delivery of care attributable to an educational program. Level 4b: focused on improvements in the health and well-being of patients as a direct result of an education initiative.
Similar to more traditional clinical research, education research needs to be performed in a scholarly fashion and shared with a wider audience. In addition to submitting research to gastroenterology journals (e.g., Gastroenterology’s Mentoring, Education, and Training Corner), education research can be submitted to education journals such as the Association of American Medical Colleges’ Academic Medicine, the Association for the Study of Medical Education’s Medical Education, the Accreditation Council for Graduate Medical Education’s Journal of Graduate Medical Education, or the European Association for Medical Education in Europe’s Medical Teacher; online education warehouses such as MedEdPORTAL (www.mededportal.org) or MERLOT (www.merlot.org); and national conferences as workshops. Also, one should keep in mind that opportunities arise on a regular basis to share educational videos or images in forums such as the American Society for Gastrointestinal Endoscopy’s video journal VideoGIE, The American Journal of Gastroenterology’s video of the month, and Clinical Gastroenterology and Hepatology’s Images of the Month.
Number 5: Ongoing professional development
Continuing Medical Education is a standard requirement to maintain an active medical license because it shows ongoing efforts to remain up-to-date with changes in medicine. Similar opportunities exist with respect to further development as an educator. Given the multitude of manners in which these opportunities can be divided, we have compiled recommendations for resources on educational scholarship based on level of experience and desired level of engagement (Table 1).
Summary
The framework provided should help guide the gastroenterologist on the path of becoming an effective clinician educator in gastroenterology. The diversity of what a career as a clinician educator can entail is unlimited. The success of the future of medical education and our careers requires not only that every clinician educator be productive, but also that each one brings a unique passion to work each day to share. The authors would like to thank all those clinician educators who contributed to our education, and look forward to learning from you in the future.
Acknowledgments
The authors thank Dr Lee Ligon, Center for Research, Innovation, and Scholarship, Department of Pediatrics, Baylor College of Medicine, for providing editorial assistance.
References
1. Sherbino, J., Frank, J.R., Snell, L. Defining the key roles and competencies of the clinician-educator of the 21st century: a national mixed-methods study. Acad Med. 2014;89:783-9.
2. Simpson, D., Fincher, R.M., Hafler, J.P., et al. Advancing educators and education by defining the components and evidence associated with educational scholarship. Med Educ. 2007;41:1002-9.
3. Rustgi, A.K. Hecht, G.A. Mentorship in academic medicine. Gastroenterology. 2011;141:789-92.
4. Farrell, S.E., Digioia, N.M., Broderick, K.B., et al. Mentoring for clinician-educators. Acad Emerg Med. 2004;11:1346-50.
5. Boyer, E.L. Scholarship reconsidered: priorities of the professoriate. Carnegie Foundation for the Advancement of Teaching, Princeton, NJ; 1990.
6. Glassick, C.E., Taylor-Huber, M., Maeroff, G.I., et al. Scholarship assessed: evaluation of the professoriate. Jossey-Bass, San Francisco; 1997.
7. Golub, R.M. Looking inward and reflecting back medical education and JAMA. JAMA. 2016;316:2200-3.
8. Crites, G.E., Gaines, J.K., Cottrell, S., et al. Medical education scholarship: an introductory guide: AMEE guide no. 89. Med Teach. 2014;36:657-74.
9. Kirkpatrick, D.L. Evaluation of training. In: R. Craig, L. Bittel (Eds.) Training and development handbook. McGraw-Hill, New York; 1967: 87-112.
10. Littlewood, S., Ypinazar, V., Margolis, S.A., et al. Early practical experience and the social responsiveness of clinical education: systematic review. BMJ. 2005;331:387-91.
Dr. Shapiro is a gastroenterology fellow in the department of medicine, section of gastroenterology; Dr. Gould Suarez is an associate professor in the department of medicine, section of gastroenterology and associate program director of the gastroenterology fellowship; and Dr. Turner is an associate professor of pediatrics, vice chair of education, associate program director for house staff education, section of academic general pediatrics, and director for research, innovation, and scholarship, Baylor College of Medicine, Houston. The authors disclose no conflicts.
For gastroenterologists who enter academic medicine, the most common career track is that of clinician educator (CE). Although most academic gastroenterologists are CEs, their career paths vary substantially, and expectations for promotion can be much less explicit compared with those of physician scientists. This delineation of different pathways in academic gastroenterology starts as early as the fellowship application process, before the implications are understood. Furthermore, many community gastroenterologists have appointments within academic medical centers, which typically fall into the realm of CEs.
A review of all gastroenterology and hepatology fellowship program websites listed on the American Gastroenterological Association website showed that 33 of 175 (18.8%) programs endorse distinctly different tracks, usually distinguishing traditional research (i.e., basic science, epidemiology, or outcomes) from clinical care of patients (i.e., clinician educator or clinical scholar). One of the most common words appearing in descriptions of both tracks was “clinical,” highlighting that a good clinician educator or researcher is, first and foremost, a good clinician.
With clinical duties requiring the majority of a CE’s time and efforts, a reasonable assumption is that CEs are clinicians who teach trainees via lectures, clinic, endoscopy, and/or inpatient rounds. Although such educational endeavors form the backbone of a CE’s scholarly activities, what constitutes scholarship for CEs is much more diverse than many people realize. The variability in what a career as a CE may look like can be both an obstacle and an opportunity. Included in the category of CE are community clinicians who have a stake in the education of residents and fellows and play an important role in trainee learning. Sherbino et al1 defined a CE as “a clinician active in health professional practice who applies theory to education practice, engages in education scholarship, and serves as a consultant to other health professionals on education issues.”
Because we recognize that many community and academic gastroenterologists spend the majority of their education efforts teaching trainees, we have made every effort to ensure that the five recommendations listed later are equally pertinent to all gastroenterologists who devote any portion of their careers to educating trainees, colleagues, allied health professionals, as well as patients. For example, a CE who primarily teaches trainees still can benefit from learning how to better document their efforts, receive mentorship as an educator, take everyday activities and convert them into scholarship, share teaching materials with broader audiences, and learn new teaching techniques without ever opening a book on education theory. For community-based physicians, this can assist in obtaining recognition from the academic centers for their teaching efforts. We hope that the five recommendations that follow will serve to guide those just setting out on the CE path, as well as for those who have trodden it for some time.
Number 1: Maintain a current curriculum vitae and teaching portfolio
All CEs must have two critical instruments to document their accomplishments to their institutions and to the field: a curriculum vitae (CV) and a teaching portfolio. These items also are very important when the time comes for promotion because they validate one’s accomplishments, both quantitatively and qualitatively. Knowing the criteria for promotion as a CE is critical for shaping one’s career, and we recommend checking with an individual’s institution for its specific requirements regarding formats for both the CV and the teaching portfolio, which typically are available from the academic promotion committee. Because most fellows and faculty are familiar with the format of a CV, we will focus on the teaching portfolio.
For most fellows and many faculty, the teaching portfolio is a new and/or less well understood entity. Unlike a CV, the teaching portfolio presents teaching activities not only as a collection or list, but also provides evidence of the influence the work has had on others, in a much more personal way. A few tips are listed on putting together a teaching portfolio. However, the most important advice we can offer is this: one should save all evidence of teaching including unsolicited letters and e-mails from learners and colleagues.
If your institution does not have a teaching portfolio template, we recommend using a pre-existing format. Several examples from academic medicine can be found on the Internet or on MedEdPORTAL, an open-access repository of educational content provided by the Association of American Medical Colleges. One such tool is the Educator Portfolio Template of the Academic Pediatric Association’s Educational Scholars Program (available: https://www.academicpeds.org/education/educator_portfolio_template.cfm). The Association of American Medical Colleges Group on Education Affairs held a consensus conference in 2006, from which five educational categories were defined: teaching, learner assessment, curriculum development, mentoring and advising, and educational leadership and administration.2 These categories can serve as an arrangement for a teaching portfolio. We also recommend that you include both educational research/scholarship and web-based educational materials such as online learning modules, YouTube videos, blogs, and wikis as a part of a teaching portfolio. For each project highlighted in the teaching portfolio, we recommend reflecting on and writing down how the project shows the quantity and quality of the work.
Quantity of work in the teaching portfolio refers to more than a mere cataloging of published peer-reviewed articles and book chapters, courses taught, presentations given, and so forth (which should be included in the CV). Instead, it documents time spent in teaching activities, how often teaching occurs, the number and types of learners involved, and how the activity fits into a training program.
Quality of work can include how innovative methods were crafted and implemented to customize teaching in creative ways to accomplish specific learning objectives. This description renders the contents of the teaching portfolio more than merely a sketch of work activities documented by numbers, and tells a story about what occurred. When documenting evidence of quality, provide comparative measures whenever possible. Quality of teaching also can be illustrated by evaluations, pretests and posttests, and as complimentary e-mails and letters from learners and other faculty members. The description of teaching activities also shows one’s flexibility as an educator, and the greater the breadth of experiences, the better. A CE also must document within the portfolio how the teaching activity drew from existing literature and best practices and/or contributed to the medical education field and its body of knowledge. Above all else, we recommend collecting evidence on teaching to both provide evidence of one’s teaching skills and to gather data on which to improve.
The teaching portfolio templates begin with a personal statement outlining why one teaches (i.e., teaching philosophy). In a teaching portfolio, it is important to include details of how impact was defined or determined with regard to teaching endeavors, how the feedback from formal evaluative processes was used to mold one’s future activities as an educator, and what strategies will be implemented to improve teaching to meet the needs of diverse and changing groups of learners.
Both the CV and teaching portfolio should be updated continually – we recommend at least quarterly (or as articles are published, courses are taught, abstracts are presented, and so forth) – to ensure that nothing is overlooked or forgotten.
Number 2: Mentors and mentees
Every CE needs to have a primary mentor, typically a more senior faculty member with an interest in and experience with mentoring, as well as a commitment to fostering the mentee’s professional growth. It may be difficult to find a mentor when starting out as a junior faculty member or when changing academic institutions. Once you have a mentor, take ownership for the success of the relationship by managing-up, by organizing all the meetings, exceeding (not just meeting) deadlines, and by communicating needs and information in a way the mentor prefers. Rustgi and Hecht3 wrap up their article on mentorship with a pathway that highlights the following components for a successful mentoring relationship: regular meetings, specific goals and measurable outcomes, manuscript and grant writing, presentation skills and efficiency, and navigating the complexities of regulatory affairs such as institutional review boards. Although many of these tenets hold true for both clinician researchers and clinician educators, Farrell et al4 offer four steps to finding a mentor for clinician educators, as follows. Step 1: self-reflection and assessment: critically assessing one’s competence as a teacher, educational administrator, or researcher; determining what prior education projects have been successful and why; and defining career goals and the current relationship to them. Step 2: identification of areas needing development: examples may include teaching skills, curriculum innovation, evaluation/assessment, educational research, time management, negotiation skills, grantsmanship, scholarly writing, and presentation skills; identify specific questions regarding the type of help needed. Step 3: matchmaking: determine qualities (personal and professional) desired in a mentor, and search for candidates with the help of colleagues. Step 4: engagement with a mentor: explain why you desire mentorship, career goals, current academic role(s), your perceived needs, and recognize and acknowledge appreciation for your mentor’s time and energy.
One caution is to avoid having too many primary mentors. A mentee may assume the perspective that it takes a village when it comes to seeking and providing mentoring. Although having clinical, research, and/or personal mentors can be helpful, having too many mentors can make it difficult to meet regularly enough to allow for the mentee–mentor relationship to grow. Instead of a network of mentors, build a web of minimentors, or coaches, to serve as consultants, coaches, and accountability partners, and tap into this network as needed. Mentors are involved longitudinally with mentees and tend to provide general career and project-specific guidance, whereas coaches tend to be involved in specific projects or areas of focus of a mentee.
In addition to having their own mentors, CEs quickly will find opportunities themselves to serve as mentors to more junior faculty, fellows, residents, and students. Indeed, one measure of a successful mentor–mentee relationship is the development of the mentee into a new mentor for future generations.
Number 3: Think broadly about scholarship
Traditionally, the definition of scholarship has been very narrow and usually is related to the number of publications and grants one receives. Beginning with Boyer’s work in 1990, the definition of scholarship has expanded at academic institutions beyond the concept of traditional research.5 Medical education scholarship most often is guided and judged by six core qualitative standards of excellence, known as “Glassick’s criteria”6: clear goals, adequate preparation, appropriate methods, significant results, effective presentation, and reflective critique. The key to scholarship is that it builds on or adds to the field, is made public, and thus available for peer-review.
CE projects can be categorized in many ways, but we recommend broadening the classic notions of research with which we have been indoctrinated. Golub’s7 2016 editorial in the Journal of the American Medical Association, “Looking Inward and Reflecting Back: Medical Education and Journal of the American Medical Association,” highlights the range of research questions and methodologies, which include ethics, behavioral psychology, diversity of patient care and the workforce, medical education research, quality and value of care, well-being of trainees and faculty, and health informatics. If one breaks down daily tasks, countless opportunities for scholarly projects will emerge. One need look no further for opportunities than the countless opportunities for quality improvement research that avail themselves daily, with examples ranging from reducing variation in cirrhosis care to improving adenoma detection rates. Quality improvement is an important method of scholarship for both academic and community-based physicians, which also can contribute toward Part IV of Maintenance of Certification requirements. CEs also can engage in educational scholarship other than research by using these same principles. To transform your teaching into scholarship you should examine the activities you perform or a problem that needs to be solved, apply information or a solution based on best practices or what is known from the literature, and then share the results/products with others (peer-review). Crites et al8 provide practical guidelines for developing education research questions, designing and implementing scholarly activities, and interpreting the scope and impact of education scholarship.
In addition, reaching beyond one’s department to other departments, as well as participating in educational scholarly activities on regional and national levels, is important as one’s career progresses. Well-connected and diverse networks are information highways by which one’s work can be amplified to achieve a greater impact, and from which many opportunities will be shared.
Number 4: Share broadly
Scholarship activities of both academic and community-based CEs can target many audiences, including medical students, residents, and fellows; faculty; other health professions; or even patients and the community. Knowing who will be the recipients or end-users can help to identify which types of projects may be most rewarding and make the greatest impact. Consider sharing curricula, evaluation tools, and other educational products with colleagues at other institutions who ask for them. Request acknowledgment for the development of the materials and ask for written feedback on how these products are being used and what impact they have had on learners.
One education model used to assess the impact and target of education interventions is known as Kirkpatrick’s9 hierarchy, which traditionally included the following four levels: reaction (level 1), learning (level 2), behavior (level 3), and results (level 4). The model has been adapted by the British Medical Journal’s Best Evidence in Medical Education collaboration to medical education with the following modifications in levels as follows.9,10 Level 1: participation: focused on learners’ views of the learning experience including content, presentation, and teaching methods. Level 2a: modification of attitudes/perceptions: focused on changes in attitudes or perceptions between participant groups toward the intervention. Level 2b: modification of knowledge/skills: for knowledge, focused on the acquisition of concepts, procedures, and principles; for skills, focused on the acquisition of problem solving, psychomotor, and social skills. Level 3: behavioral change: focused on the transfer of learning to the workplace or willingness of learners to apply new knowledge and skills. Level 4a: change in organizational practice: focused on wider changes in the organization or delivery of care attributable to an educational program. Level 4b: focused on improvements in the health and well-being of patients as a direct result of an education initiative.
Similar to more traditional clinical research, education research needs to be performed in a scholarly fashion and shared with a wider audience. In addition to submitting research to gastroenterology journals (e.g., Gastroenterology’s Mentoring, Education, and Training Corner), education research can be submitted to education journals such as the Association of American Medical Colleges’ Academic Medicine, the Association for the Study of Medical Education’s Medical Education, the Accreditation Council for Graduate Medical Education’s Journal of Graduate Medical Education, or the European Association for Medical Education in Europe’s Medical Teacher; online education warehouses such as MedEdPORTAL (www.mededportal.org) or MERLOT (www.merlot.org); and national conferences as workshops. Also, one should keep in mind that opportunities arise on a regular basis to share educational videos or images in forums such as the American Society for Gastrointestinal Endoscopy’s video journal VideoGIE, The American Journal of Gastroenterology’s video of the month, and Clinical Gastroenterology and Hepatology’s Images of the Month.
Number 5: Ongoing professional development
Continuing Medical Education is a standard requirement to maintain an active medical license because it shows ongoing efforts to remain up-to-date with changes in medicine. Similar opportunities exist with respect to further development as an educator. Given the multitude of manners in which these opportunities can be divided, we have compiled recommendations for resources on educational scholarship based on level of experience and desired level of engagement (Table 1).
Summary
The framework provided should help guide the gastroenterologist on the path of becoming an effective clinician educator in gastroenterology. The diversity of what a career as a clinician educator can entail is unlimited. The success of the future of medical education and our careers requires not only that every clinician educator be productive, but also that each one brings a unique passion to work each day to share. The authors would like to thank all those clinician educators who contributed to our education, and look forward to learning from you in the future.
Acknowledgments
The authors thank Dr Lee Ligon, Center for Research, Innovation, and Scholarship, Department of Pediatrics, Baylor College of Medicine, for providing editorial assistance.
References
1. Sherbino, J., Frank, J.R., Snell, L. Defining the key roles and competencies of the clinician-educator of the 21st century: a national mixed-methods study. Acad Med. 2014;89:783-9.
2. Simpson, D., Fincher, R.M., Hafler, J.P., et al. Advancing educators and education by defining the components and evidence associated with educational scholarship. Med Educ. 2007;41:1002-9.
3. Rustgi, A.K. Hecht, G.A. Mentorship in academic medicine. Gastroenterology. 2011;141:789-92.
4. Farrell, S.E., Digioia, N.M., Broderick, K.B., et al. Mentoring for clinician-educators. Acad Emerg Med. 2004;11:1346-50.
5. Boyer, E.L. Scholarship reconsidered: priorities of the professoriate. Carnegie Foundation for the Advancement of Teaching, Princeton, NJ; 1990.
6. Glassick, C.E., Taylor-Huber, M., Maeroff, G.I., et al. Scholarship assessed: evaluation of the professoriate. Jossey-Bass, San Francisco; 1997.
7. Golub, R.M. Looking inward and reflecting back medical education and JAMA. JAMA. 2016;316:2200-3.
8. Crites, G.E., Gaines, J.K., Cottrell, S., et al. Medical education scholarship: an introductory guide: AMEE guide no. 89. Med Teach. 2014;36:657-74.
9. Kirkpatrick, D.L. Evaluation of training. In: R. Craig, L. Bittel (Eds.) Training and development handbook. McGraw-Hill, New York; 1967: 87-112.
10. Littlewood, S., Ypinazar, V., Margolis, S.A., et al. Early practical experience and the social responsiveness of clinical education: systematic review. BMJ. 2005;331:387-91.
Dr. Shapiro is a gastroenterology fellow in the department of medicine, section of gastroenterology; Dr. Gould Suarez is an associate professor in the department of medicine, section of gastroenterology and associate program director of the gastroenterology fellowship; and Dr. Turner is an associate professor of pediatrics, vice chair of education, associate program director for house staff education, section of academic general pediatrics, and director for research, innovation, and scholarship, Baylor College of Medicine, Houston. The authors disclose no conflicts.
Making social media work for your practice
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.
Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
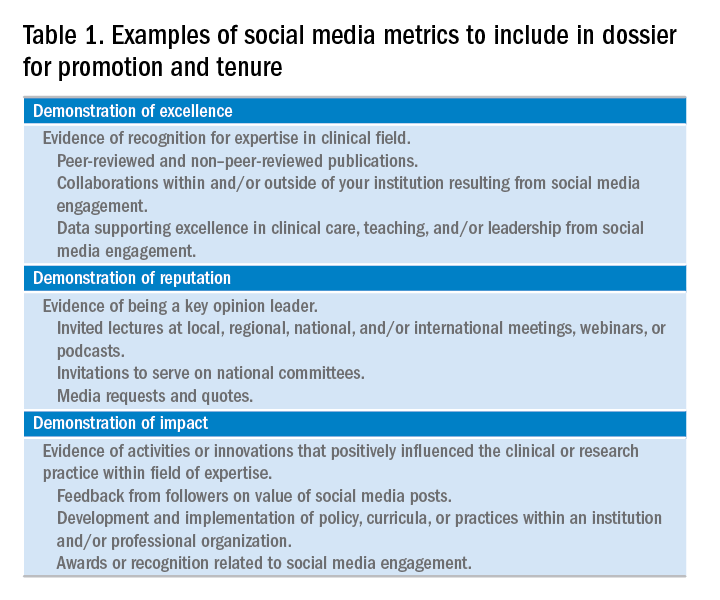
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.

Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.

Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
New opportunities for gastroenterology leadership in the evolving payment reform landscape
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.
Constructing an inflammatory bowel disease patient–centered medical home
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).
Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.
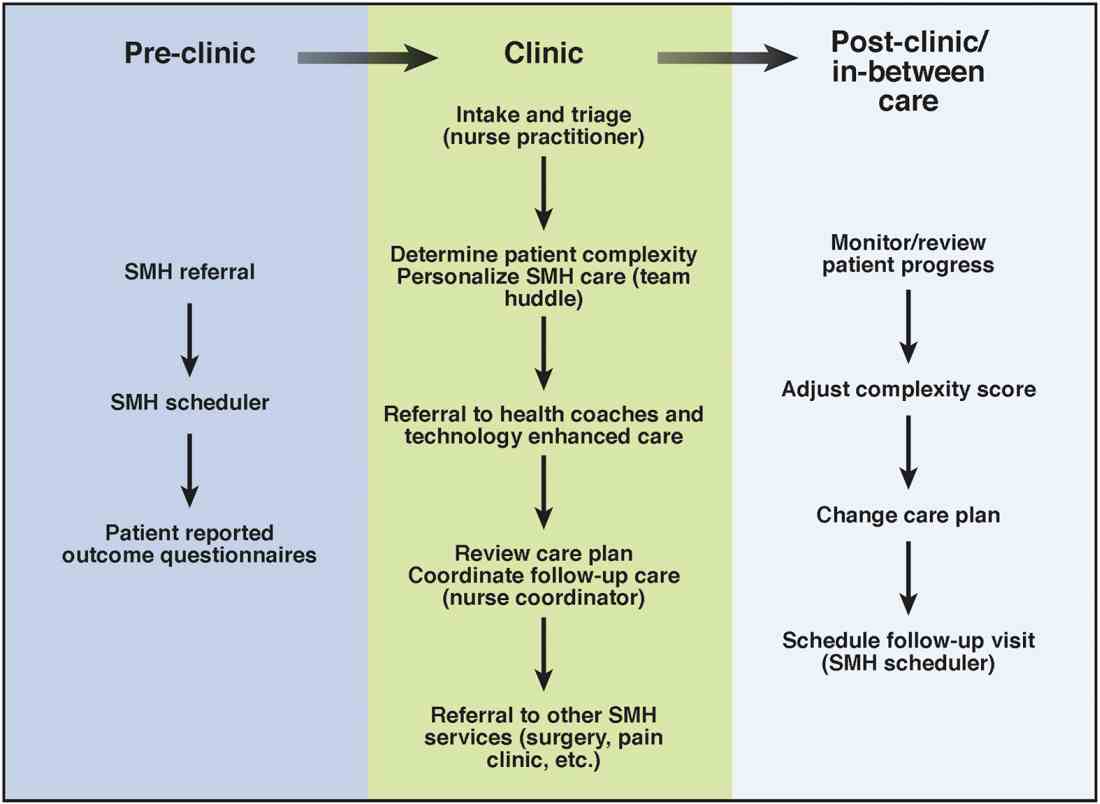
Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).

Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.

Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.
Inflammatory bowel diseases (IBDs) including Crohn’s disease and ulcerative colitis are life-long chronic diseases with high morbidity. There has been remarkable progress in the understanding of disease pathophysiology, leading to new medical therapies and surgical approaches for the management of IBD. These trends have resulted in a marked increase in the cost of IBD care, with current estimates ranging from $14 to $31 billion in both direct and indirect costs in the United States.1
IBD patients have unique behavioral, preventive, and therapeutic care requirements.2,3 Because of the complexity of care, there is a large degree of segmentation and fragmentation of IBD management across health care systems and among multiple providers. This siloed approach often falls short of seamless, efficient, high-quality, patient-centered care.
To address the increasing costs and fragmentation of chronic disease management, population-based health care has emerged as a new concept with an emphasis on reward for value, not volume. Two such examples of population-based health care include accountable care organizations and patient-centered medical homes. This concept relies on the development of new payment models and shifts the risk to the providers.4,5 Primary care providers play a central coordinating role in these new models.6,7 However, the role of specialists is less well defined, with limited sharing of risk for the care and costs of populations.
The IBD specialty medical home (SMH) implemented at the University of Pittsburgh Medical Center (UPMC) is an example of a new model of care. The IBD SMH is constructed to align incentives and provide up-front resources to manage a population of patients with IBD optimally – including treatment of their inflammatory disease, coexisting pain, and psychological issues.8-10 In the case of the IBD SMH, the gastroenterologist is the principal provider for a cohort of IBD patients. The gastroenterologist is responsible for the coordination and management of health care of this population and places the IBD patient at the center of the medical universe.
In this article, we draw from our rich partnership between the UPMC Health Plan (HP) and Health System to describe the construction and deployment of the IBD SMH. Although this model is new and we still are learning, we already have seen an improvement in the overall quality of life, decreased utilization, and reduction in total cost of care for this IBD SMH population.
Constructing an IBD medical home: where to begin?
In conjunction with the UPMC HP, we designed and established an IBD patient-centered SMH, designated in July 2015 as UPMC Total Care–Inflammatory Bowel Disease.11 The development of the medical home was facilitated by our unique integrated delivery and finance system. The UPMC HP provided important utilization data on their IBD population, which allowed for focused enrollment of the highest-utilizer patients. In addition, the UPMC HP funded positions that we hired directly as employees of our IBD SMH. These positions included the following: two nurse coordinators, two certified nurse practitioners, a dietitian, a social worker, and a psychiatrist. The UPMC HP also provided their own HP employees to work with our IBD SMH: The rare and chronic disease team included two nurses and a social worker who made house calls for a select group of patients (identified based on the frequency of their health care use). The HP also provided health coaches who worked directly with our patients on lifestyle modifications, such as smoking cessation and exercise programs. Finally, the UPMC HP worked with the IBD SMH to provide support for a variety of operational functions. Examples of these important efforts included data analytics through their department of health economics, regular collaboration to assist the provider team in modifying the program, publicizing the IBD SMH to their members, and facilitating approval of IBD medications through their pharmacy department.
We acknowledge that the development and implementation of an IBD SMH will vary from region to region and depend on the relationship of payers and providers. Thus, the blueprint of our UPMC IBD Medical Home may not be replicated readily in other centers or regions. However, there are several core elements that we believe are necessary in constructing any SMH: 1) a payer willing to partner with the provider, 2) a patient population with specific characteristics, 3) a physician champion, and 4) prespecified goals and measures of success.
Payer or health plan
A SMH is based on the premise that providers and payers working together can achieve more efficient, high-quality care for patients than either party working alone. Payers have essential resources for infrastructure support, preventive services delivery, marketing and engagement expertise, large databases for risk stratification and gap closure, and care management capacity to be a valuable partner. In the short term, philanthropy, grants, and crowd-sourcing options can be used to provide initial support for components of the SMH; however, these rarely are sustainable long-term options. Thus, the most critical collaboration necessary to considering a SMH is between payer(s) (insurance company or health plan) and the specialty provider.
Ideally, the local environment should consist of a single or a few large payers to ease SMH implementation. UPMC is a large integrated delivery (25 hospitals and more than 600 clinics) and financing system (more than 3 million members and is the dominant payer in the region), with a history of leveraging payer–provider partnerships to achieve better patient care, education, and research, and thus served as an ideal collaborator in the design and launch of the IBD SMH. Most physicians in the United States do not work in an integrated payer–provider health delivery system, and partnering with a large regional payer with an interest in specialty population-based chronic care is reasonable for constructing an SMH in your medical neighborhood.
Patient population
In addition to having a collaborative health plan with large population coverage, there must exist a substantial IBD population managed by gastroenterologists. There must be a sufficient number of high-utilizer, high-cost members to justify up-front capital expenditure and return on investment. To determine the feasibility and utility of creating an IBD SMH at UPMC, we collected baseline data on the following: 1) the number of IBD patients within our IBD center and health plan, 2) a hotspotting analysis for our Pennsylvania counties, and 3) health care utilization of the IBD population of interest. At the time of the SMH inception, there were 6,319 Crohn’s disease and ulcerative colitis patients (including all insurance plans) in our center, with more than 3,500 members insured by our HP. There was a 30% increase in new IBD patients to our center in the 3 years before starting the IBD SMH, and the HP had a 27% increase in overall IBD members. Based on a regional hotspotting analysis, $24.3 million of the annual total of $36.9 million was related to hospitalization costs from our IBD patients. The high-utilizer patients accounted for most of the total cost of care for our HP; 16% accounted for 48% of the per-member per-month cost and 29% accounted for 79% of the total annual cost. These baseline data supported justification for an IBD SMH.
Although there is no absolute minimum number of members (patients) required, and the SMH model can be scaled to various IBD populations, we believe that at least 1,000 patients covered by a single insurer must exist. The justification for the 1,000 patients is an estimate of the number of high-utilizer patients who would be required to justify a cost savings, and ultimately a return on investment. We calculated that at least 300 high-utilizer patients would need to be included in our IBD SMH to show a reduction in health care utilization and total cost of care. Therefore, if we assume that approximately 30% of any chronic disease population drives the majority of cost and represents the highest utilizers, we estimated that at least 1,000 patients should be covered by a single insurer.
For development of an SMH, there are two approaches that may be taken: Design the medical home for the entire Health Plan’s population of patients with the disease of interest, or focus only on the high-utilizing, most expensive patients. The latter will include a more complex and challenging cohort of patients, but likely will provide the opportunity to show a reduction in utilization and total cost of care than a broader all-comers population approach.
Physician champions
A successful SMH requires a physician (or health care provider) champion. IBD care within the SMH is unique and distinct from gastroenterologists’ classic training and specialty care. In addition to addressing the biologic disease, the emphasis is on whole-patient care: preventive care, behavioral medicine, socioeconomic considerations of the patient, and provision of care for nongastrointestinal symptoms and diseases. In an SMH, the specialist must be willing to incorporate and address all facets of health care to improve patient outcomes.
Goals and measures of success
To ensure successful deployment of an SMH, it is important to establish shared payer–provider goals and metrics during the construction phase of the medical home. These goals should include an enrollment target number for each year, quality improvement metrics, patient experience outcomes, and metrics for a reduction in health care utilization and total cost of care. Examples of our IBD SMH year 1 and year 2 goals are outlined in Supplementary Table 1 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026). In the first year of our IBD SMH, we were able to achieve our goals, and publication of our results is forthcoming. We have enrolled more than 325 patients, retained 90%, reduced emergency room visits and hospitalizations by 50%, and significantly improved quality of life. Most of our patients have been assigned an HP coach and use the electronic medical portal to communicate with the medical home. Our patient satisfaction for physician communication was 99%.
Key components of the IBD medical home
Based on our experience, we believe the following are key components of a successful IBD SMH: 1) team-based care with physician extenders, nurse coordinators, schedulers, social workers, and dietitians as essential members of the IBD SMH; 2) effective care coordination to reduce barriers to comprehensive biopsychosocial care; 3) tracking of process and outcome metrics of interest; 4) appropriate use of technology to enhance clinical care; and 5) care access (e.g., open-access appointments), after-hours care, and follow-up care after emergency room visits and hospitalizations (Table 1).

Although the eventual goal of an IBD SMH is to consolidate health care for all IBD patients, the initial launch stages are more likely to succeed if the SMH focuses on the subgroup of IBD patients who use health care excessively, often in an unplanned fashion (e.g., emergency department visits or hospitalizations). In conjunction with a payer, it is easy to identify the most costly IBD patients in a cohort. For example, for initial enrollment, the UPMC IBD SMH selected patients between the ages of 18 and 55 years, with confirmed Crohn’s disease or ulcerative colitis, and evidence that IBD was a primary driver of patients’ health care utilization; the latter was defined if the majority of health care expenditures in the prior year was related to IBD (as judged by International Classification of Diseases, 9th and 10th revisions, primary and secondary diagnoses).
Team-based care
A central component to our IBD SMH was the creation of an integrated team. Supplementary Table 2 (at http://dx.doi.org/10.1016/j.cgh.2017.05.026) describes various positions that are vital for a successful SMH. For a team approach to be most effective, there needs to be clear definitions or roles and role overlaps so that team members can work as a cohesive, organized, and efficient unit. Physician extenders are critical to the model’s success and are trained to make routine IBD care decisions, provide basic primary care, and coordinate care with the gastroenterologist to meet patient needs. The staff-to-patient ratio requirements may vary from region to region and from SMH to SMH. The nurse coordinators and physician extenders assume the burden of day-to-day patient care, and are supervised by the gastroenterologist and psychiatrist. In our UPMC IBD SMH, the ratio of one nurse coordinator and one certified nurse practitioner per 500 patients is sufficient. In addition, one social worker, one dietitian, one scheduler, one gastroenterologist, and one psychiatrist per 1,000 patients is our current model. To date, we have enrolled more than 500 patients, and through funding from our UPMC HP, we have just hired our second nurse coordinator and second nurse practitioner in anticipation of 1,000 patients by year 4.

Care coordination and incorporation of technology
The team composition is organized to provide tiered care for optimal efficiency. For such a stepped care model to be effective and scalable, two components are essential. The first component is a care coordination system that allows for the reliable classification of the biological, psychological, social, and health systems barriers faced by patients. To this end, our SMH developed an IBD-specific complexity grid (Supplementary Table 3; at http://dx.doi.org/10.1016/j.cgh.2017.05.026) that was derived from a primary care model.12 The second component is the use of technology-enhanced care to scale delivery of services in a population health model. Examples of technology in our SMH include the use of telemedicine/telepsychiatry by secure video, health coach virtual visits, remote monitoring, and provider-assisted behavioral interventions that patients can access on their smart phones.
New payment models for specialty medical homes
The SMH transitions away from relative value unit–based reimbursement and toward a value-based paradigm. In the SMH, the gastroenterologist serves as the principal medical provider for the IBD patient. Both providers and payers will be able to refer patients to the SMH. Data on quality metrics will be tracked and physician extenders and nurse coordinators will help ensure that goals are met. Quality improvement, preventive medicine, telemedicine, and point-of-contact mental health care will replace the volume-based relative value unit system.
Alternative payment models will be required to support the SMH. Because of the novel nature of the SMH, the optimal payment model has yet to be determined, but probably will include either a shared savings or global cap approach, with an emphasis on the total cost of care reduction. This means that the specialist in the SMH must be aware of all care, and the cost of care, that the patient receives. Biologics and other IBD therapy costs are high and will continue to increase. The sustainable model must be sufficiently supple to not disincentivize the provider to use proven and effective, albeit expensive, therapy for patients who need it most. A close working relationship between the SMH providers and the health plan chief pharmacy officer will be essential. We expect that appropriate use of medications will lead to a medical cost offset with improved IBD outcomes, a reduction in health care utilization, and optimized work and life productivity.
Conclusions
In new models of care, specialty providers partner with payers in a patient-centered system to provide principal care for patients with chronic diseases, including IBD, in an effort to reduce costs and provide efficient, high-quality care. These models will require close collaborations with payers, a sufficiently large patient population, a physician champion, and a multidisciplinary staff targeting various aspects of health care. Successful implementation of such models will help reduce costs of care while improving the patient-centered experience and outcomes.
Supplementary material
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.05.026.
References
1. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22(Suppl):s51-60.
2. Mikocka-Walus A., Knowles S.R., Keefer L., et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752-62.
3. Regueiro M., Greer J.B., Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430-9.e4.
4. Silow-Carroll S., Edwards J.N., Rodin D. How Colorado, Minnesota, and Vermont are reforming care delivery and payment to improve health and lower costs. Issue Brief. (Commonw Fund) 2013;10:1-9.
5. Fogelman C., Gates T. A primary care perspective on U.S. health care: part 2: thinking globally, acting locally. J Lancaster Gen Hospital. 2013;8:101-5.
6. Rosenthal M.B., Sinaiko A.D., Eastman D., et al. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967-73.
7. Friedberg M.W., Rosenthal M.B., Werner R.M., et al. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175:1362-8.
8. Fernandes S.M., Sanders L.M. Patient-centered medical home for patients with complex congenital heart disease. Curr Opin Pediatr. 2015;27:581-6.
9. Mikocka-Walus A.A., Andrews J.M., Bernstein C.N., et al. Integrated models of care in managing inflammatory bowel disease: a discussion. Inflamm Bowel Dis. 2012;18:1582-7.
10. Kessler R., Miller B.F., Kelly M., et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. J Am Board Fam Med. 2014;27:637-44.
11. Regueiro M.D., McAnallen S.E., Greer J.B., et al. The inflammatory bowel disease specialty medical home: a new model of patient centered care. Inflamm Bowel Dis. 2016;22:1971-80.
12. Lobo E., Ventura T., Navio M., et al. Identification of components of health complexity on internal medicine units by means of the INTERMED method. Int J Clin Pract. 2015;69:1377-86.
Dr. Regueiro and Dr. Click are in the division of gastroenterology, hepatology and nutrition, University of Pittsburgh Medical Center; Ms. Holder, Dr. Shrank, and Ms. McAnAllen are in the Insurance Services Division, University of Pittsburgh Medical Center, and Dr. Szigethy is in the Department of Psychiatry, University of Pittsburgh School of Medicine. Dr. Regueiro serves as a consultant for, on advisory boards for, and receives research support from Abbvie, Janssen, and Takeda; and Dr. Szigethy serves as a consultant for Abbvie. The remaining authors disclose no conflicts.
Cultivating competencies for value-based care
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.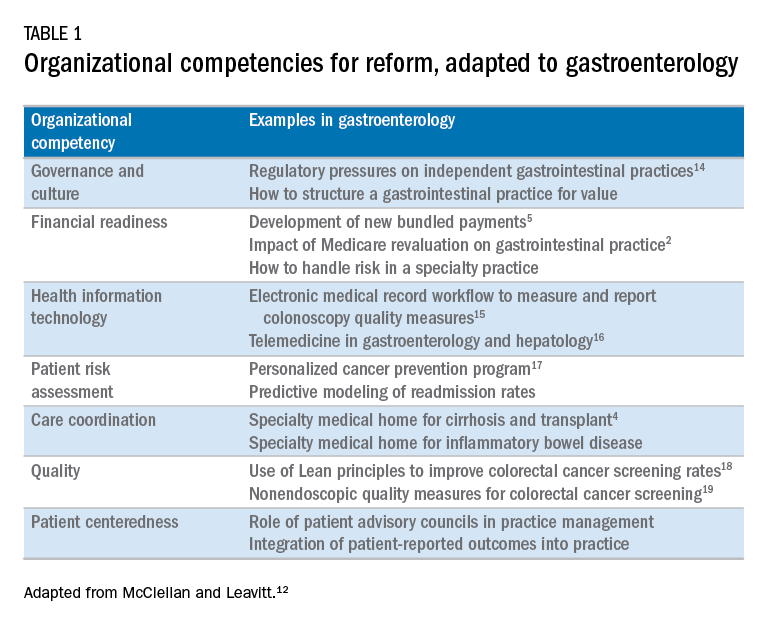
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
From Obamacare to Trumpcare – implications for gastroenterologists
The June issue of CGH was the final column under my management. I have enjoyed the opportunity to provide you with information about practice management and health care reform. I also have enjoyed working with the Clinical Gastroenterology and Hepatology board of editors, and Erin Landis and Brook Simpson from AGA headquarters. Beginning in July 2017, this section will become the responsibility of Ziad Gellad, MD, MPH, AGAF, from Duke University. I have worked with Ziad for many years, and he serves on my board of editors for GI & Hepatology News. I have great confidence in his knowledge and ability.
During the last 5 years, we have published 58 columns beginning with an article where I made several broad predictions. I have tried to present important concepts and management tools related to private and academic clinical practice, health care reform, and health economics. This article was written in early January 2017 just before the inauguration of Donald Trump. As I wrote, we did not know the full extent or the pace of “Repeal and Replace,” as Obamacare becomes Trumpcare (www.healthaffairs.org/obamacare-to-trumpcare).
The extent of current Republican control of federal and state governments is unprecedented in modern political history. Per Newt Gingrich (The Economist, Jan. 7, 2017, p. 25), this will be the third attempt, after Ronald Reagan’s election in 1980 and Gingrich’s “Contract with America” in 1994, to break free from a “Big Government” mindset initiated by Franklin Roosevelt’s New Deal. In this article, I will speculate how a right-leaning shift in American health care policy might impact the business model of gastroenterology. No matter how government regulations or funds flow change, we (physicians) will ultimately be responsible for digestive care provided to our patients. In the words of Martin Luther King Jr. (as he paraphrased Theodore Parker), “The arc of the moral universe is long, but it bends toward justice.” What is remembered by fewer people, however, are words he then added during his speeches: “but only if we march.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
The first column was published in July 2012.1 I wrote about five dominant themes that would alter our gastroenterology practices in the ensuing years. They were 1) an increasing requirement for us to demonstrate value, 2) the need to think about population management in addition to individual patient care, 3) consolidation that would occur at all levels of health care delivery, 4) increasing cost pressure, and 5) how medical decisions would be linked to reimbursement (now called value-based payment). I fully expected the Patient Protection and Affordable Care Act (ACA) would shape the health care landscape for the rest of our careers. After the article’s publication, I was invited to speak about health care reform at many academic centers and private practices. My last talk before the election was in Pasadena, Calif. (Oct. 28, 2016) where I confidently spoke about the implications of President Clinton’s cementing ACA into the fabric of U.S. medicine.
Donald Trump is now the 45th President of the United States. Republicans hold a 52-48 majority in the Senate and a 241-194 majority in the House. As of January 2017, one Supreme Court seat was available, and three more may open because of retirements (Justice Ginsburg is 83 years old, Justice Kennedy is 80, and Justice Breyer is 78). Republicans control all three branches of government in 25 states and dominate in 8 others. Conservative politicians control a large majority of county and city boards.
Until this year, Republicans have controlled all three branches of government only twice since 1945 (modern political history), and only once (George Bush in 2005) did the president have a Senate majority.2 With his win, Mr. Trump can lead a conservative revolution to reverse key initiatives begun when the Democratic Party held majority power. Repeal of the ACA, signed into legislation on March 23, 2010, is the Republican Party’s top priority.
Equally important, Congress can alter previously implemented federal regulations. Each year about 3,000 regulations are written by federal agencies that act with authority delegated by Congress (albeit Congress retains power to overturn them). Regulations are published in the Federal Register as preliminary rules during each year, and Final Rules are published after a public comment period and implemented shortly thereafter. Regulations carry the force of law and are codified in the Code of Federal Regulations. The Code of Federal Regulations is divided into 50 sections (Titles), with Title 42 (Public Health) and Title 45 (Public Welfare) most pertinent to us.
Other policies are created through executive orders, issued by the president (federal) or governors (states), without involvement of legislative or judicial branches (they were not mentioned in the Constitution, by the way). Executive orders issued by President Obama could, theoretically, be overturned by new executive orders.
Repeal and replace
Destruction of the ACA is a top priority of President Trump and Republican leaders of both houses of Congress. The ACA was a Democratic bill (passed with no Republican support), although it had many similarities to previous Republican legislative ideas dating from 1993.3
Although outright repeal could be blocked by a Democratic filibuster, the law could be drastically modified through budget reconciliation whose passage takes only a simple Senate majority. Thus, a simple budget-related bill could serve as a vehicle to defund many parts of ACA, including money for Medicaid expansion, insurance risk corridors, money to offset out-of-pocket expenses and individual premium subsidies, for example.4,5
There would be substantial problems if ACA were repealed even with a 2- or 3-year delay, a scenario proposed to provide time for a replacement bill. On Jan. 4, 2017, the House Republican Study Committee introduced the American Health Care Reform Act (AHCRA) as a replacement proposal, with the stipulation that ACA would be repealed as of Jan. 1, 2018. This initial bill hinted at Republican intent and was detailed in a Health Affairs blog.6 Importantly, there were distinct similarities between this and prior Republican proposals put forward by Representative Tom Price (nominated to head the Department of Health & Human Services under President Trump) and Speaker of the House Paul Ryan.7,8
Consistently, Republicans have advocated for expansion of health savings accounts, altering the tax code to allow individuals to deduct health insurance premiums, establishment of association risk pools, imposition of malpractice limits, protections for people with preexisting conditions, and further restrictions on abortion coverage. The AHCRA changes financial subsidies for purchasing insurance from a tax credit (which can be paid to people even if they do not pay taxes) to a tax deduction (only applicable to people who pay taxes). Analysis of a similar proposal made by President Trump during the campaign found that this plan would increase the number of uninsured people by more than 15 million.
If ACA is repealed, effects would be broader than just factors related to insurance coverage.9 ACA provides for preventive care (including colonoscopy) without copays, education of additional medical personnel, closing the donut hole for Medicare Part D (medications), approval of generic biologics, and Medicaid expansions, among other initiatives. If ACA were defunded without restoring pre-ACA support for Disproportion Share Hospital charity care, research, and graduate medical education, then safety-net hospitals and many academic medical centers (AMCs) could face enormous funding cuts.10 Defunding Medicaid expansion would adversely affect states in many ways, as pointed out by Ayanian et al.11 Medicaid expansion had broad economic impact in states that accepted federal money to expand. In Michigan for example, 30,900 jobs were added to the state in 2016 because of Medicaid expansion, with two-thirds outside of the health care industry. President Obama defined his view about the effects of ACA repeal in the New England Journal of Medicine.12
Lessons learned
Economic principles and unique characteristics of United States health care help explain why solutions to its high cost and uneven coverage are so difficult to achieve. These include higher prices for goods in the United States compared with other countries, variation in price (unrelated to quality), restraints on government price negotiations, inefficiencies due to variation in size of delivery systems, and “moral hazard” related to rich insurance coverage, which are some of the factors that doom any simple solutions. These are reviewed by Victor Fuchs13 in an excellent article in Annals of Internal Medicine. Payment methods for health care services also distort resource use and efficiencies. Understanding the eight basic payment methodologies in health care and current predictions about future health care spending will be important in shaping reimbursement policies.14,15
Disruptions in health care are unpopular and, as Uve Reinardt stated: “Our health care financing system will always remain a horrendous mess and a fountain for such dismay among the providers of health care as well as among patients.”4 Lessons to inform the next iteration of health care policy, learned from the 2009-2010 experience, might be as follows:
1. If a bill is to be passed, the president must personally lead in explaining the bill to the public in simple terms.
2. Even the threat of repeal may disrupt the current market and force insurance companies to exit quickly.
3. Coverage must be affordable to individuals, state budgets, and health care providers. Because expansion states saw positive impacts to state budgets8 and mental health and substance abuse services became part of Medicaid benefits, how will a replacement bill maintain coverage and compensate for new state moneys used now for other imperatives such as education and infrastructure?
4. Health care is like a massive cargo ship, not a sports car, so a bill to replace the ACA may take a long time (and might never be passed).
5. Health care is intensely personal, so it will always be politically charged.
Ultimately, physicians will need to make strategic guesses and rapid adjustments to sustain financial viability and provide high-value care. Strategies differ depending on your practice situation. Keep in mind the five principles listed in the opening paragraph of this article. It is likely that the most important principle to factor into your practice strategy is continuing reduction in reimbursements. No matter what model is adopted to reform the ACA, the financial pot (Medicare, Medicaid, commercial insurance, bundled payments, fee-for-service payments) will be reduced, and the number of uninsured patients will increase. How would you change your practice if Medicare was your best payer (“manage to Medicare”)?
Independent practices
Physicians in small- to medium-size independent practices continue to struggle with reducing reimbursements, reporting burdens, increasing overhead expenses, crushing regulatory requirements, and provider burnout. Trumpcare will favor small practices more than Obamacare from a policy (not necessarily a financial) perspective. Regulations on small business and reporting burdens may ease, but the move toward value-based reimbursement as outlined in the MACRA (passed with overwhelming bipartisan support) will not end.16 Practices in small communities continue to thrive because they give excellent care with limited competition and low overhead. Some practices in suburban and urban centers struggle because payers tend to favor (with enhanced managed care rates) larger practices and health systems. Large, horizontally integrated, efficient gastroenterology practices will continue to thrive because they can develop a “must-have” position with payers. Building remote patient monitoring, teleconsulting, and capabilities around value demonstration will be strategically advantageous.
Options for independent physicians include 1) maintaining status quo, 2) retiring, or 3) exiting the independent business model through a practice sale. Traditionally, physicians who wanted to sell their practices turned to hospitals or health systems. Recently, a physician-run model funded by venture capital has emerged where reduced overhead (through centralization of services) is combined with enhanced power during payer negotiations (because of scale). This model has allowed practices to merge into a physician organization and remain free from health system employment.17
Large health systems
Physicians employed by large health systems, whether they are nonprofit, for-profit, or AMCs, will see their future tied directly to health system success. If bundled payment, alternative payment, and capitation models of health care financing continue to grow in popularity, then success will be determined by a health system’s market share and its ability to form true clinical integration. In a capitated environment, expansion of market share (especially of relatively healthy patients) will help support margins. However, financial success will come from a system’s ability to manage high-cost patients, those 5% of patients who consume 50% of health care resources.18
Hospitals with a financially challenged patient base (safety-net hospitals) will have enormous financial pressures going forward. Repeal of ACA without restoration of pre-ACA funding will affect directly the financial health of systems including AMCs. AMCs and other health systems will be forced to reduce fixed overhead, enhance productivity of faculty, and restrict nonfunded activities (teaching for example). Although most AMCs are now in an active acquisition mode, this strategy is naturally limited by the number of remaining acquisition targets. Traditional high managed care rates enjoyed by AMCs will shrink, as will federal research funding (which typically comes with high indirect financial support). Health systems and GI societies will need to dedicate much more attention to state policy makers as Trumpcare progresses.
Finally, all providers will need to manage the business implications of retail health. As people assume higher deductibles and copays and health savings accounts grow, patients will change their patterns of purchasing services. Reputation counts for less when people are facing large price differences, so attention to patient-centric amenities, price, patient engagement, and patient satisfaction will become even more important.
Conclusion
The United States has undergone a massive and rapid political transformation. The mandate felt by conservative politicians, perhaps not supported by numbers, will carry a conservative platform forward. In areas where progressive Democrats emphasized federal power and socialized regulation (religion, education, civil rights, income security, and health policy), conservatives will transfer decision power as much as possible to states, local communities, and individuals. Maintaining the concept of “health as a right” will test the conscience of all of us.
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Gill KE. Visual guide: the balance of power between Congress and the Presidency (1901-2016). Wired Pen. November 2016. Available from http://wiredpen.com/resources/political-commentary-and-analysis/a-visual-guide-balance-of-power-congress-presidency/. Accessed Dec. 30, 2016.
3. Mertens M. Chart: comparing health reform bills – Democrats and Republicans 2009, Republics 1993. Kaiser Health News. Feb. 24, 2010. Available from http://khn.org/022310-bill-comparison/. Accessed Jan. 8, 2017.
4. Hotchkiss M. Q&A: what a Trump presidency means for the Affordable Care Act. Nov. 16, 2016 News at Princeton. Princeton University. Available from https://www.princeton.edu/main/news/archive/S47/93/09C11/index.xml?section=topstories. Accessed Dec. 30, 2016.
5. Jost T. Taking stock of health reform: where we’ve been, where we’re going. Health Affairs Blog. Available from http://healthaffairs.org/blog/2016/12/06/taking-stock-of-health-reform-where-weve-been-where-were-going/. Accessed Dec. 30, 2016.
6. Jost T. The Republican Study Committee’s ACA replacement proposal (updated). Health Affairs Blog. Available from http://healthaffairs.org/blog/2017/01/05/the-republican-study-committees-aca-replacement-proposal/. Accessed Jan. 10, 2017.
7. Price T. Empowering patients first. Available from http://tomprice.house.gov/sites/tomprice.house.gov/files/HR%202300%20Empowering%20Patients%20First%20Act%202015.pdf. Accessed Dec. 30, 2016.
8. Ryan P. A better way. Available from https://abetterway.speaker.gov/_assets/pdf/ABetterWay-HealthCare-PolicyPaper.pdf. Accessed Dec. 30, 2016.
9. Oberlander, J. The end of Obamacare. N Engl J Med. 2017;376:1-3.
10. Goodnough A. Hospitals in safety net brace for health care law’s repeal. New York Times. Dec. 28, 2016. Available from http://www.nytimes.com/2016/12/28/health/hospitals-medicaid-obamacare-trump.html?smprod=nytcore-iphone&smid=nytcore-iphone-share&_r=0. Accessed Jan. 10, 2017.
11. Ayanian, J.Z., Ehrlich, G.M., Grimes, D.R., et al. Economic effects of Medicaid expansion in Michigan. N Engl J Med. 2017;376:407-10.
12. Obama, B.H. Repealing the ACA without a replacement: the risks to American health care. N Engl J Med. 2017;376:297-9.
13. Fuchs, V.R. Major concepts of health economics. Ann Intern Med. 2015;162:380-3.
14. Quinn, K. The 8 basic payment methods in health care. Ann Intern Med. 2015;163:300-6.
15. Schoenman JA. A detailed look at US health care spending: a presentation from the National Institute for Health Care Management (NIHCM). Oct. 25, 2012. Available from http://www.nihcm.org/images/stories/Health_care_spending_slides_-_MILI_-_Schoenman.pdf. Accessed Dec. 30, 2016.
16. Allen, J.I., Allen, C.C., Brill, J.V. Gastroenterology 2020: no time for WIMPs. Gastroenterology. 2016;150:295-9.
17. Sciacca R. Weekly Byte: GI roundtable and navigating uncharted waters in health care. The PMD Blog. Available from https://www.pmd.com/blog/post/weekly-byte-gi-roundtable-and-navigating-uncharted-waters-in-health-care. Accessed Jan. 10, 2017.
18. Powers, B.W., Chaguturu, S.K. ACOs and high-cost patients. N Engl J Med. 2016;374:203-5.
Dr. Allen is professor of medicine, University of Michigan School of Medicine, Institute for Health Care Policy and Innovations, and associate medical director of Network Strategy and Business Development – Michigan Medicine, Ann Arbor. He discloses no conflicts.
The June issue of CGH was the final column under my management. I have enjoyed the opportunity to provide you with information about practice management and health care reform. I also have enjoyed working with the Clinical Gastroenterology and Hepatology board of editors, and Erin Landis and Brook Simpson from AGA headquarters. Beginning in July 2017, this section will become the responsibility of Ziad Gellad, MD, MPH, AGAF, from Duke University. I have worked with Ziad for many years, and he serves on my board of editors for GI & Hepatology News. I have great confidence in his knowledge and ability.
During the last 5 years, we have published 58 columns beginning with an article where I made several broad predictions. I have tried to present important concepts and management tools related to private and academic clinical practice, health care reform, and health economics. This article was written in early January 2017 just before the inauguration of Donald Trump. As I wrote, we did not know the full extent or the pace of “Repeal and Replace,” as Obamacare becomes Trumpcare (www.healthaffairs.org/obamacare-to-trumpcare).
The extent of current Republican control of federal and state governments is unprecedented in modern political history. Per Newt Gingrich (The Economist, Jan. 7, 2017, p. 25), this will be the third attempt, after Ronald Reagan’s election in 1980 and Gingrich’s “Contract with America” in 1994, to break free from a “Big Government” mindset initiated by Franklin Roosevelt’s New Deal. In this article, I will speculate how a right-leaning shift in American health care policy might impact the business model of gastroenterology. No matter how government regulations or funds flow change, we (physicians) will ultimately be responsible for digestive care provided to our patients. In the words of Martin Luther King Jr. (as he paraphrased Theodore Parker), “The arc of the moral universe is long, but it bends toward justice.” What is remembered by fewer people, however, are words he then added during his speeches: “but only if we march.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
The first column was published in July 2012.1 I wrote about five dominant themes that would alter our gastroenterology practices in the ensuing years. They were 1) an increasing requirement for us to demonstrate value, 2) the need to think about population management in addition to individual patient care, 3) consolidation that would occur at all levels of health care delivery, 4) increasing cost pressure, and 5) how medical decisions would be linked to reimbursement (now called value-based payment). I fully expected the Patient Protection and Affordable Care Act (ACA) would shape the health care landscape for the rest of our careers. After the article’s publication, I was invited to speak about health care reform at many academic centers and private practices. My last talk before the election was in Pasadena, Calif. (Oct. 28, 2016) where I confidently spoke about the implications of President Clinton’s cementing ACA into the fabric of U.S. medicine.
Donald Trump is now the 45th President of the United States. Republicans hold a 52-48 majority in the Senate and a 241-194 majority in the House. As of January 2017, one Supreme Court seat was available, and three more may open because of retirements (Justice Ginsburg is 83 years old, Justice Kennedy is 80, and Justice Breyer is 78). Republicans control all three branches of government in 25 states and dominate in 8 others. Conservative politicians control a large majority of county and city boards.
Until this year, Republicans have controlled all three branches of government only twice since 1945 (modern political history), and only once (George Bush in 2005) did the president have a Senate majority.2 With his win, Mr. Trump can lead a conservative revolution to reverse key initiatives begun when the Democratic Party held majority power. Repeal of the ACA, signed into legislation on March 23, 2010, is the Republican Party’s top priority.
Equally important, Congress can alter previously implemented federal regulations. Each year about 3,000 regulations are written by federal agencies that act with authority delegated by Congress (albeit Congress retains power to overturn them). Regulations are published in the Federal Register as preliminary rules during each year, and Final Rules are published after a public comment period and implemented shortly thereafter. Regulations carry the force of law and are codified in the Code of Federal Regulations. The Code of Federal Regulations is divided into 50 sections (Titles), with Title 42 (Public Health) and Title 45 (Public Welfare) most pertinent to us.
Other policies are created through executive orders, issued by the president (federal) or governors (states), without involvement of legislative or judicial branches (they were not mentioned in the Constitution, by the way). Executive orders issued by President Obama could, theoretically, be overturned by new executive orders.
Repeal and replace
Destruction of the ACA is a top priority of President Trump and Republican leaders of both houses of Congress. The ACA was a Democratic bill (passed with no Republican support), although it had many similarities to previous Republican legislative ideas dating from 1993.3
Although outright repeal could be blocked by a Democratic filibuster, the law could be drastically modified through budget reconciliation whose passage takes only a simple Senate majority. Thus, a simple budget-related bill could serve as a vehicle to defund many parts of ACA, including money for Medicaid expansion, insurance risk corridors, money to offset out-of-pocket expenses and individual premium subsidies, for example.4,5
There would be substantial problems if ACA were repealed even with a 2- or 3-year delay, a scenario proposed to provide time for a replacement bill. On Jan. 4, 2017, the House Republican Study Committee introduced the American Health Care Reform Act (AHCRA) as a replacement proposal, with the stipulation that ACA would be repealed as of Jan. 1, 2018. This initial bill hinted at Republican intent and was detailed in a Health Affairs blog.6 Importantly, there were distinct similarities between this and prior Republican proposals put forward by Representative Tom Price (nominated to head the Department of Health & Human Services under President Trump) and Speaker of the House Paul Ryan.7,8
Consistently, Republicans have advocated for expansion of health savings accounts, altering the tax code to allow individuals to deduct health insurance premiums, establishment of association risk pools, imposition of malpractice limits, protections for people with preexisting conditions, and further restrictions on abortion coverage. The AHCRA changes financial subsidies for purchasing insurance from a tax credit (which can be paid to people even if they do not pay taxes) to a tax deduction (only applicable to people who pay taxes). Analysis of a similar proposal made by President Trump during the campaign found that this plan would increase the number of uninsured people by more than 15 million.
If ACA is repealed, effects would be broader than just factors related to insurance coverage.9 ACA provides for preventive care (including colonoscopy) without copays, education of additional medical personnel, closing the donut hole for Medicare Part D (medications), approval of generic biologics, and Medicaid expansions, among other initiatives. If ACA were defunded without restoring pre-ACA support for Disproportion Share Hospital charity care, research, and graduate medical education, then safety-net hospitals and many academic medical centers (AMCs) could face enormous funding cuts.10 Defunding Medicaid expansion would adversely affect states in many ways, as pointed out by Ayanian et al.11 Medicaid expansion had broad economic impact in states that accepted federal money to expand. In Michigan for example, 30,900 jobs were added to the state in 2016 because of Medicaid expansion, with two-thirds outside of the health care industry. President Obama defined his view about the effects of ACA repeal in the New England Journal of Medicine.12
Lessons learned
Economic principles and unique characteristics of United States health care help explain why solutions to its high cost and uneven coverage are so difficult to achieve. These include higher prices for goods in the United States compared with other countries, variation in price (unrelated to quality), restraints on government price negotiations, inefficiencies due to variation in size of delivery systems, and “moral hazard” related to rich insurance coverage, which are some of the factors that doom any simple solutions. These are reviewed by Victor Fuchs13 in an excellent article in Annals of Internal Medicine. Payment methods for health care services also distort resource use and efficiencies. Understanding the eight basic payment methodologies in health care and current predictions about future health care spending will be important in shaping reimbursement policies.14,15
Disruptions in health care are unpopular and, as Uve Reinardt stated: “Our health care financing system will always remain a horrendous mess and a fountain for such dismay among the providers of health care as well as among patients.”4 Lessons to inform the next iteration of health care policy, learned from the 2009-2010 experience, might be as follows:
1. If a bill is to be passed, the president must personally lead in explaining the bill to the public in simple terms.
2. Even the threat of repeal may disrupt the current market and force insurance companies to exit quickly.
3. Coverage must be affordable to individuals, state budgets, and health care providers. Because expansion states saw positive impacts to state budgets8 and mental health and substance abuse services became part of Medicaid benefits, how will a replacement bill maintain coverage and compensate for new state moneys used now for other imperatives such as education and infrastructure?
4. Health care is like a massive cargo ship, not a sports car, so a bill to replace the ACA may take a long time (and might never be passed).
5. Health care is intensely personal, so it will always be politically charged.
Ultimately, physicians will need to make strategic guesses and rapid adjustments to sustain financial viability and provide high-value care. Strategies differ depending on your practice situation. Keep in mind the five principles listed in the opening paragraph of this article. It is likely that the most important principle to factor into your practice strategy is continuing reduction in reimbursements. No matter what model is adopted to reform the ACA, the financial pot (Medicare, Medicaid, commercial insurance, bundled payments, fee-for-service payments) will be reduced, and the number of uninsured patients will increase. How would you change your practice if Medicare was your best payer (“manage to Medicare”)?
Independent practices
Physicians in small- to medium-size independent practices continue to struggle with reducing reimbursements, reporting burdens, increasing overhead expenses, crushing regulatory requirements, and provider burnout. Trumpcare will favor small practices more than Obamacare from a policy (not necessarily a financial) perspective. Regulations on small business and reporting burdens may ease, but the move toward value-based reimbursement as outlined in the MACRA (passed with overwhelming bipartisan support) will not end.16 Practices in small communities continue to thrive because they give excellent care with limited competition and low overhead. Some practices in suburban and urban centers struggle because payers tend to favor (with enhanced managed care rates) larger practices and health systems. Large, horizontally integrated, efficient gastroenterology practices will continue to thrive because they can develop a “must-have” position with payers. Building remote patient monitoring, teleconsulting, and capabilities around value demonstration will be strategically advantageous.
Options for independent physicians include 1) maintaining status quo, 2) retiring, or 3) exiting the independent business model through a practice sale. Traditionally, physicians who wanted to sell their practices turned to hospitals or health systems. Recently, a physician-run model funded by venture capital has emerged where reduced overhead (through centralization of services) is combined with enhanced power during payer negotiations (because of scale). This model has allowed practices to merge into a physician organization and remain free from health system employment.17
Large health systems
Physicians employed by large health systems, whether they are nonprofit, for-profit, or AMCs, will see their future tied directly to health system success. If bundled payment, alternative payment, and capitation models of health care financing continue to grow in popularity, then success will be determined by a health system’s market share and its ability to form true clinical integration. In a capitated environment, expansion of market share (especially of relatively healthy patients) will help support margins. However, financial success will come from a system’s ability to manage high-cost patients, those 5% of patients who consume 50% of health care resources.18
Hospitals with a financially challenged patient base (safety-net hospitals) will have enormous financial pressures going forward. Repeal of ACA without restoration of pre-ACA funding will affect directly the financial health of systems including AMCs. AMCs and other health systems will be forced to reduce fixed overhead, enhance productivity of faculty, and restrict nonfunded activities (teaching for example). Although most AMCs are now in an active acquisition mode, this strategy is naturally limited by the number of remaining acquisition targets. Traditional high managed care rates enjoyed by AMCs will shrink, as will federal research funding (which typically comes with high indirect financial support). Health systems and GI societies will need to dedicate much more attention to state policy makers as Trumpcare progresses.
Finally, all providers will need to manage the business implications of retail health. As people assume higher deductibles and copays and health savings accounts grow, patients will change their patterns of purchasing services. Reputation counts for less when people are facing large price differences, so attention to patient-centric amenities, price, patient engagement, and patient satisfaction will become even more important.
Conclusion
The United States has undergone a massive and rapid political transformation. The mandate felt by conservative politicians, perhaps not supported by numbers, will carry a conservative platform forward. In areas where progressive Democrats emphasized federal power and socialized regulation (religion, education, civil rights, income security, and health policy), conservatives will transfer decision power as much as possible to states, local communities, and individuals. Maintaining the concept of “health as a right” will test the conscience of all of us.
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Gill KE. Visual guide: the balance of power between Congress and the Presidency (1901-2016). Wired Pen. November 2016. Available from http://wiredpen.com/resources/political-commentary-and-analysis/a-visual-guide-balance-of-power-congress-presidency/. Accessed Dec. 30, 2016.
3. Mertens M. Chart: comparing health reform bills – Democrats and Republicans 2009, Republics 1993. Kaiser Health News. Feb. 24, 2010. Available from http://khn.org/022310-bill-comparison/. Accessed Jan. 8, 2017.
4. Hotchkiss M. Q&A: what a Trump presidency means for the Affordable Care Act. Nov. 16, 2016 News at Princeton. Princeton University. Available from https://www.princeton.edu/main/news/archive/S47/93/09C11/index.xml?section=topstories. Accessed Dec. 30, 2016.
5. Jost T. Taking stock of health reform: where we’ve been, where we’re going. Health Affairs Blog. Available from http://healthaffairs.org/blog/2016/12/06/taking-stock-of-health-reform-where-weve-been-where-were-going/. Accessed Dec. 30, 2016.
6. Jost T. The Republican Study Committee’s ACA replacement proposal (updated). Health Affairs Blog. Available from http://healthaffairs.org/blog/2017/01/05/the-republican-study-committees-aca-replacement-proposal/. Accessed Jan. 10, 2017.
7. Price T. Empowering patients first. Available from http://tomprice.house.gov/sites/tomprice.house.gov/files/HR%202300%20Empowering%20Patients%20First%20Act%202015.pdf. Accessed Dec. 30, 2016.
8. Ryan P. A better way. Available from https://abetterway.speaker.gov/_assets/pdf/ABetterWay-HealthCare-PolicyPaper.pdf. Accessed Dec. 30, 2016.
9. Oberlander, J. The end of Obamacare. N Engl J Med. 2017;376:1-3.
10. Goodnough A. Hospitals in safety net brace for health care law’s repeal. New York Times. Dec. 28, 2016. Available from http://www.nytimes.com/2016/12/28/health/hospitals-medicaid-obamacare-trump.html?smprod=nytcore-iphone&smid=nytcore-iphone-share&_r=0. Accessed Jan. 10, 2017.
11. Ayanian, J.Z., Ehrlich, G.M., Grimes, D.R., et al. Economic effects of Medicaid expansion in Michigan. N Engl J Med. 2017;376:407-10.
12. Obama, B.H. Repealing the ACA without a replacement: the risks to American health care. N Engl J Med. 2017;376:297-9.
13. Fuchs, V.R. Major concepts of health economics. Ann Intern Med. 2015;162:380-3.
14. Quinn, K. The 8 basic payment methods in health care. Ann Intern Med. 2015;163:300-6.
15. Schoenman JA. A detailed look at US health care spending: a presentation from the National Institute for Health Care Management (NIHCM). Oct. 25, 2012. Available from http://www.nihcm.org/images/stories/Health_care_spending_slides_-_MILI_-_Schoenman.pdf. Accessed Dec. 30, 2016.
16. Allen, J.I., Allen, C.C., Brill, J.V. Gastroenterology 2020: no time for WIMPs. Gastroenterology. 2016;150:295-9.
17. Sciacca R. Weekly Byte: GI roundtable and navigating uncharted waters in health care. The PMD Blog. Available from https://www.pmd.com/blog/post/weekly-byte-gi-roundtable-and-navigating-uncharted-waters-in-health-care. Accessed Jan. 10, 2017.
18. Powers, B.W., Chaguturu, S.K. ACOs and high-cost patients. N Engl J Med. 2016;374:203-5.
Dr. Allen is professor of medicine, University of Michigan School of Medicine, Institute for Health Care Policy and Innovations, and associate medical director of Network Strategy and Business Development – Michigan Medicine, Ann Arbor. He discloses no conflicts.
The June issue of CGH was the final column under my management. I have enjoyed the opportunity to provide you with information about practice management and health care reform. I also have enjoyed working with the Clinical Gastroenterology and Hepatology board of editors, and Erin Landis and Brook Simpson from AGA headquarters. Beginning in July 2017, this section will become the responsibility of Ziad Gellad, MD, MPH, AGAF, from Duke University. I have worked with Ziad for many years, and he serves on my board of editors for GI & Hepatology News. I have great confidence in his knowledge and ability.
During the last 5 years, we have published 58 columns beginning with an article where I made several broad predictions. I have tried to present important concepts and management tools related to private and academic clinical practice, health care reform, and health economics. This article was written in early January 2017 just before the inauguration of Donald Trump. As I wrote, we did not know the full extent or the pace of “Repeal and Replace,” as Obamacare becomes Trumpcare (www.healthaffairs.org/obamacare-to-trumpcare).
The extent of current Republican control of federal and state governments is unprecedented in modern political history. Per Newt Gingrich (The Economist, Jan. 7, 2017, p. 25), this will be the third attempt, after Ronald Reagan’s election in 1980 and Gingrich’s “Contract with America” in 1994, to break free from a “Big Government” mindset initiated by Franklin Roosevelt’s New Deal. In this article, I will speculate how a right-leaning shift in American health care policy might impact the business model of gastroenterology. No matter how government regulations or funds flow change, we (physicians) will ultimately be responsible for digestive care provided to our patients. In the words of Martin Luther King Jr. (as he paraphrased Theodore Parker), “The arc of the moral universe is long, but it bends toward justice.” What is remembered by fewer people, however, are words he then added during his speeches: “but only if we march.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
The first column was published in July 2012.1 I wrote about five dominant themes that would alter our gastroenterology practices in the ensuing years. They were 1) an increasing requirement for us to demonstrate value, 2) the need to think about population management in addition to individual patient care, 3) consolidation that would occur at all levels of health care delivery, 4) increasing cost pressure, and 5) how medical decisions would be linked to reimbursement (now called value-based payment). I fully expected the Patient Protection and Affordable Care Act (ACA) would shape the health care landscape for the rest of our careers. After the article’s publication, I was invited to speak about health care reform at many academic centers and private practices. My last talk before the election was in Pasadena, Calif. (Oct. 28, 2016) where I confidently spoke about the implications of President Clinton’s cementing ACA into the fabric of U.S. medicine.
Donald Trump is now the 45th President of the United States. Republicans hold a 52-48 majority in the Senate and a 241-194 majority in the House. As of January 2017, one Supreme Court seat was available, and three more may open because of retirements (Justice Ginsburg is 83 years old, Justice Kennedy is 80, and Justice Breyer is 78). Republicans control all three branches of government in 25 states and dominate in 8 others. Conservative politicians control a large majority of county and city boards.
Until this year, Republicans have controlled all three branches of government only twice since 1945 (modern political history), and only once (George Bush in 2005) did the president have a Senate majority.2 With his win, Mr. Trump can lead a conservative revolution to reverse key initiatives begun when the Democratic Party held majority power. Repeal of the ACA, signed into legislation on March 23, 2010, is the Republican Party’s top priority.
Equally important, Congress can alter previously implemented federal regulations. Each year about 3,000 regulations are written by federal agencies that act with authority delegated by Congress (albeit Congress retains power to overturn them). Regulations are published in the Federal Register as preliminary rules during each year, and Final Rules are published after a public comment period and implemented shortly thereafter. Regulations carry the force of law and are codified in the Code of Federal Regulations. The Code of Federal Regulations is divided into 50 sections (Titles), with Title 42 (Public Health) and Title 45 (Public Welfare) most pertinent to us.
Other policies are created through executive orders, issued by the president (federal) or governors (states), without involvement of legislative or judicial branches (they were not mentioned in the Constitution, by the way). Executive orders issued by President Obama could, theoretically, be overturned by new executive orders.
Repeal and replace
Destruction of the ACA is a top priority of President Trump and Republican leaders of both houses of Congress. The ACA was a Democratic bill (passed with no Republican support), although it had many similarities to previous Republican legislative ideas dating from 1993.3
Although outright repeal could be blocked by a Democratic filibuster, the law could be drastically modified through budget reconciliation whose passage takes only a simple Senate majority. Thus, a simple budget-related bill could serve as a vehicle to defund many parts of ACA, including money for Medicaid expansion, insurance risk corridors, money to offset out-of-pocket expenses and individual premium subsidies, for example.4,5
There would be substantial problems if ACA were repealed even with a 2- or 3-year delay, a scenario proposed to provide time for a replacement bill. On Jan. 4, 2017, the House Republican Study Committee introduced the American Health Care Reform Act (AHCRA) as a replacement proposal, with the stipulation that ACA would be repealed as of Jan. 1, 2018. This initial bill hinted at Republican intent and was detailed in a Health Affairs blog.6 Importantly, there were distinct similarities between this and prior Republican proposals put forward by Representative Tom Price (nominated to head the Department of Health & Human Services under President Trump) and Speaker of the House Paul Ryan.7,8
Consistently, Republicans have advocated for expansion of health savings accounts, altering the tax code to allow individuals to deduct health insurance premiums, establishment of association risk pools, imposition of malpractice limits, protections for people with preexisting conditions, and further restrictions on abortion coverage. The AHCRA changes financial subsidies for purchasing insurance from a tax credit (which can be paid to people even if they do not pay taxes) to a tax deduction (only applicable to people who pay taxes). Analysis of a similar proposal made by President Trump during the campaign found that this plan would increase the number of uninsured people by more than 15 million.
If ACA is repealed, effects would be broader than just factors related to insurance coverage.9 ACA provides for preventive care (including colonoscopy) without copays, education of additional medical personnel, closing the donut hole for Medicare Part D (medications), approval of generic biologics, and Medicaid expansions, among other initiatives. If ACA were defunded without restoring pre-ACA support for Disproportion Share Hospital charity care, research, and graduate medical education, then safety-net hospitals and many academic medical centers (AMCs) could face enormous funding cuts.10 Defunding Medicaid expansion would adversely affect states in many ways, as pointed out by Ayanian et al.11 Medicaid expansion had broad economic impact in states that accepted federal money to expand. In Michigan for example, 30,900 jobs were added to the state in 2016 because of Medicaid expansion, with two-thirds outside of the health care industry. President Obama defined his view about the effects of ACA repeal in the New England Journal of Medicine.12
Lessons learned
Economic principles and unique characteristics of United States health care help explain why solutions to its high cost and uneven coverage are so difficult to achieve. These include higher prices for goods in the United States compared with other countries, variation in price (unrelated to quality), restraints on government price negotiations, inefficiencies due to variation in size of delivery systems, and “moral hazard” related to rich insurance coverage, which are some of the factors that doom any simple solutions. These are reviewed by Victor Fuchs13 in an excellent article in Annals of Internal Medicine. Payment methods for health care services also distort resource use and efficiencies. Understanding the eight basic payment methodologies in health care and current predictions about future health care spending will be important in shaping reimbursement policies.14,15
Disruptions in health care are unpopular and, as Uve Reinardt stated: “Our health care financing system will always remain a horrendous mess and a fountain for such dismay among the providers of health care as well as among patients.”4 Lessons to inform the next iteration of health care policy, learned from the 2009-2010 experience, might be as follows:
1. If a bill is to be passed, the president must personally lead in explaining the bill to the public in simple terms.
2. Even the threat of repeal may disrupt the current market and force insurance companies to exit quickly.
3. Coverage must be affordable to individuals, state budgets, and health care providers. Because expansion states saw positive impacts to state budgets8 and mental health and substance abuse services became part of Medicaid benefits, how will a replacement bill maintain coverage and compensate for new state moneys used now for other imperatives such as education and infrastructure?
4. Health care is like a massive cargo ship, not a sports car, so a bill to replace the ACA may take a long time (and might never be passed).
5. Health care is intensely personal, so it will always be politically charged.
Ultimately, physicians will need to make strategic guesses and rapid adjustments to sustain financial viability and provide high-value care. Strategies differ depending on your practice situation. Keep in mind the five principles listed in the opening paragraph of this article. It is likely that the most important principle to factor into your practice strategy is continuing reduction in reimbursements. No matter what model is adopted to reform the ACA, the financial pot (Medicare, Medicaid, commercial insurance, bundled payments, fee-for-service payments) will be reduced, and the number of uninsured patients will increase. How would you change your practice if Medicare was your best payer (“manage to Medicare”)?
Independent practices
Physicians in small- to medium-size independent practices continue to struggle with reducing reimbursements, reporting burdens, increasing overhead expenses, crushing regulatory requirements, and provider burnout. Trumpcare will favor small practices more than Obamacare from a policy (not necessarily a financial) perspective. Regulations on small business and reporting burdens may ease, but the move toward value-based reimbursement as outlined in the MACRA (passed with overwhelming bipartisan support) will not end.16 Practices in small communities continue to thrive because they give excellent care with limited competition and low overhead. Some practices in suburban and urban centers struggle because payers tend to favor (with enhanced managed care rates) larger practices and health systems. Large, horizontally integrated, efficient gastroenterology practices will continue to thrive because they can develop a “must-have” position with payers. Building remote patient monitoring, teleconsulting, and capabilities around value demonstration will be strategically advantageous.
Options for independent physicians include 1) maintaining status quo, 2) retiring, or 3) exiting the independent business model through a practice sale. Traditionally, physicians who wanted to sell their practices turned to hospitals or health systems. Recently, a physician-run model funded by venture capital has emerged where reduced overhead (through centralization of services) is combined with enhanced power during payer negotiations (because of scale). This model has allowed practices to merge into a physician organization and remain free from health system employment.17
Large health systems
Physicians employed by large health systems, whether they are nonprofit, for-profit, or AMCs, will see their future tied directly to health system success. If bundled payment, alternative payment, and capitation models of health care financing continue to grow in popularity, then success will be determined by a health system’s market share and its ability to form true clinical integration. In a capitated environment, expansion of market share (especially of relatively healthy patients) will help support margins. However, financial success will come from a system’s ability to manage high-cost patients, those 5% of patients who consume 50% of health care resources.18
Hospitals with a financially challenged patient base (safety-net hospitals) will have enormous financial pressures going forward. Repeal of ACA without restoration of pre-ACA funding will affect directly the financial health of systems including AMCs. AMCs and other health systems will be forced to reduce fixed overhead, enhance productivity of faculty, and restrict nonfunded activities (teaching for example). Although most AMCs are now in an active acquisition mode, this strategy is naturally limited by the number of remaining acquisition targets. Traditional high managed care rates enjoyed by AMCs will shrink, as will federal research funding (which typically comes with high indirect financial support). Health systems and GI societies will need to dedicate much more attention to state policy makers as Trumpcare progresses.
Finally, all providers will need to manage the business implications of retail health. As people assume higher deductibles and copays and health savings accounts grow, patients will change their patterns of purchasing services. Reputation counts for less when people are facing large price differences, so attention to patient-centric amenities, price, patient engagement, and patient satisfaction will become even more important.
Conclusion
The United States has undergone a massive and rapid political transformation. The mandate felt by conservative politicians, perhaps not supported by numbers, will carry a conservative platform forward. In areas where progressive Democrats emphasized federal power and socialized regulation (religion, education, civil rights, income security, and health policy), conservatives will transfer decision power as much as possible to states, local communities, and individuals. Maintaining the concept of “health as a right” will test the conscience of all of us.
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Gill KE. Visual guide: the balance of power between Congress and the Presidency (1901-2016). Wired Pen. November 2016. Available from http://wiredpen.com/resources/political-commentary-and-analysis/a-visual-guide-balance-of-power-congress-presidency/. Accessed Dec. 30, 2016.
3. Mertens M. Chart: comparing health reform bills – Democrats and Republicans 2009, Republics 1993. Kaiser Health News. Feb. 24, 2010. Available from http://khn.org/022310-bill-comparison/. Accessed Jan. 8, 2017.
4. Hotchkiss M. Q&A: what a Trump presidency means for the Affordable Care Act. Nov. 16, 2016 News at Princeton. Princeton University. Available from https://www.princeton.edu/main/news/archive/S47/93/09C11/index.xml?section=topstories. Accessed Dec. 30, 2016.
5. Jost T. Taking stock of health reform: where we’ve been, where we’re going. Health Affairs Blog. Available from http://healthaffairs.org/blog/2016/12/06/taking-stock-of-health-reform-where-weve-been-where-were-going/. Accessed Dec. 30, 2016.
6. Jost T. The Republican Study Committee’s ACA replacement proposal (updated). Health Affairs Blog. Available from http://healthaffairs.org/blog/2017/01/05/the-republican-study-committees-aca-replacement-proposal/. Accessed Jan. 10, 2017.
7. Price T. Empowering patients first. Available from http://tomprice.house.gov/sites/tomprice.house.gov/files/HR%202300%20Empowering%20Patients%20First%20Act%202015.pdf. Accessed Dec. 30, 2016.
8. Ryan P. A better way. Available from https://abetterway.speaker.gov/_assets/pdf/ABetterWay-HealthCare-PolicyPaper.pdf. Accessed Dec. 30, 2016.
9. Oberlander, J. The end of Obamacare. N Engl J Med. 2017;376:1-3.
10. Goodnough A. Hospitals in safety net brace for health care law’s repeal. New York Times. Dec. 28, 2016. Available from http://www.nytimes.com/2016/12/28/health/hospitals-medicaid-obamacare-trump.html?smprod=nytcore-iphone&smid=nytcore-iphone-share&_r=0. Accessed Jan. 10, 2017.
11. Ayanian, J.Z., Ehrlich, G.M., Grimes, D.R., et al. Economic effects of Medicaid expansion in Michigan. N Engl J Med. 2017;376:407-10.
12. Obama, B.H. Repealing the ACA without a replacement: the risks to American health care. N Engl J Med. 2017;376:297-9.
13. Fuchs, V.R. Major concepts of health economics. Ann Intern Med. 2015;162:380-3.
14. Quinn, K. The 8 basic payment methods in health care. Ann Intern Med. 2015;163:300-6.
15. Schoenman JA. A detailed look at US health care spending: a presentation from the National Institute for Health Care Management (NIHCM). Oct. 25, 2012. Available from http://www.nihcm.org/images/stories/Health_care_spending_slides_-_MILI_-_Schoenman.pdf. Accessed Dec. 30, 2016.
16. Allen, J.I., Allen, C.C., Brill, J.V. Gastroenterology 2020: no time for WIMPs. Gastroenterology. 2016;150:295-9.
17. Sciacca R. Weekly Byte: GI roundtable and navigating uncharted waters in health care. The PMD Blog. Available from https://www.pmd.com/blog/post/weekly-byte-gi-roundtable-and-navigating-uncharted-waters-in-health-care. Accessed Jan. 10, 2017.
18. Powers, B.W., Chaguturu, S.K. ACOs and high-cost patients. N Engl J Med. 2016;374:203-5.
Dr. Allen is professor of medicine, University of Michigan School of Medicine, Institute for Health Care Policy and Innovations, and associate medical director of Network Strategy and Business Development – Michigan Medicine, Ann Arbor. He discloses no conflicts.
Digital cohorts within the social mediome to circumvent conventional research challenges?
We are becoming comfortable with the concept of a sharing economy, where resources are shared among many individuals using online forums. Whether activities involve sharing rides (Uber, Lyft, and others), accommodations (Airbnb), or information (social media), underlying attributes include reduced transactional costs, enhanced information transparency, dynamic feedback, and socialization of opportunity. As health care systems realize that they are changing from direct-to-business to a direct-to-customer model, their ability to connect directly with individuals will become a foundational strategy.
This month’s column introduces us to social media as a research tool. Information derived from social media sites can be harvested for critical clinical information (the Centers for Disease and Control and Prevention tracks the spread of influenza using social media analytic tools), research data (patient preferences), and as a recruitment method for clinical studies. Kulanthaivel and colleagues have described their experiences and literature review to help us imagine new ways to collect data at markedly reduced transaction costs (compared to a formal clinical trial). While there are many cautions about the use of social media in your practice or research, we are only beginning to understand its potential.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Medical knowledge, culminating from the collection and translation of patient data, is the primary objective of the clinical research paradigm. The successful conduct of this traditional model has become even more challenging with expansion of costs and a dwindling research infrastructure. Beyond systemic issues, conventional research methods are burdened further by minimal patient engagement, inadequate staffing, and geographic limitations to recruitment. Clinical research also has failed to keep pace with patient demands, and the limited scope of well-funded, disease-specific investigations have left many patients feeling disenfranchised. Social media venues may represent a viable option to surpass these current and evolving barriers when used as an adjunctive approach to traditional clinical investigation.
Advantages and pitfalls in social media research
SM is a new frontier containing a wide spectrum of clinical and qualitative data from connected users (patients). Collection and examination of either individuals’ or groups’ SM information use can provide insight into qualitative life experiences, just as analysis of biologic samples can enable dissection of genetic disease underpinnings. This mediome is analogous to the human genome, both in content and utility.1 Analyzing data streams from SM for interpersonal interactions, message content, and even frequency can provide digital investigators with volumes of information that otherwise would remain unattainable.
Several limitations and potential risks of SM for medical research should be addressed, including the possible compromise of privacy and confidentiality, the use and dissemination of medical advice and information, potential demographic biases, and a required trust of the investigator by patients. Many of these challenges can be similar to traditional methods, however, as in the conventional model, careful management can drastically reduce unwanted study issues.
The risk of Health Insurance Portability and Accountability Act violations must be considered seriously in the context of patient–researcher interactions on SM. Because of the relatively public nature of these venues, patient confidentiality may be at risk if patients choose to divulge personal medical information. However, if proper protective measures are taken to ensure that the venue is secure (e.g., a private or closed group on Facebook or a by-invitation-only online Internet forum), and the researcher vets all patients who request entrance into the group, this risk may be minimized. Moreover, to further reduce any legal liability, the researcher should not provide any medical advice to patients who participate in a SM study. The drive to provide medical direction in study patients with clinical need may be strong because collaborative relationships between investigator and patients are likely to form. Furthermore, digital access to investigators on SM commonly becomes easy for patients. Safe approaches to communication could include redirecting patients to consult with their own doctor for advice, unbiased dissemination of disease-specific educational materials, or depiction of only institutional review board–approved study materials.7,8
The perception that only younger populations use SM may appear to be a significant limitation for its implementation in clinical research. However, this limitation is rapidly becoming less significant because recent studies have shown that the use of SM has become increasingly common among older adults. As of 2014, more than half of the US adult population used Facebook, including 73% and 63% of Internet-using adults ages 30–49 and 50–64 years, respectively.10 SM may not be suitable for all diseases, however, there is likely significant demographic overlap for many disease populations.
Finally, it is imperative for researchers to gain the trust of patients on SM to effectively use these venues for research purposes. Because patient–researcher interaction does not occur face-to-face on these platforms, gaining the trust of patients may be more difficult than it would be in a clinical setting. Thus, patient–patient and patient–researcher communications within SM platforms must be cultivated carefully to instill participant confidence in the research being performed on their behalf. One of the authors (C.L.) has established an SM educational model for this exchange.4 Specifically, he provides patients with a distillation of current field research by posting updates in a research-specific Facebook group and on Twitter. This model not only empowers patients with disease education, it also solidifies the importance of patient investment in disease-specific research. Furthermore, invested patients bring ideas to research, take a more educated and proactive role in their care team, and, ultimately, return to seek more study involvement.
Social media in rare disease research
Rare diseases (conditions with a prevalence of less than 200,000 patients in North America), in particular, are prime for high-yield results and community impact using novel SM approaches. This is the result of established digital support groups, publications with historically low study numbers, and few focused investigators. Several studies of rare diseases have shown considerable advantages of using SM as a study tool. For instance, an existing neuroendocrine cervical cancer Facebook support group recently was used to recruit a geographically widespread cohort of patients with this rare cancer. Through an online survey posted in the Facebook group, patients were able to provide specific information on their treatment, disease, and symptom history, current disease status, and quality of life, including various psychological factors. Without the use of SM, collecting this information would have been virtually impossible because the patients were treated at 51 cancer centers across the country.14
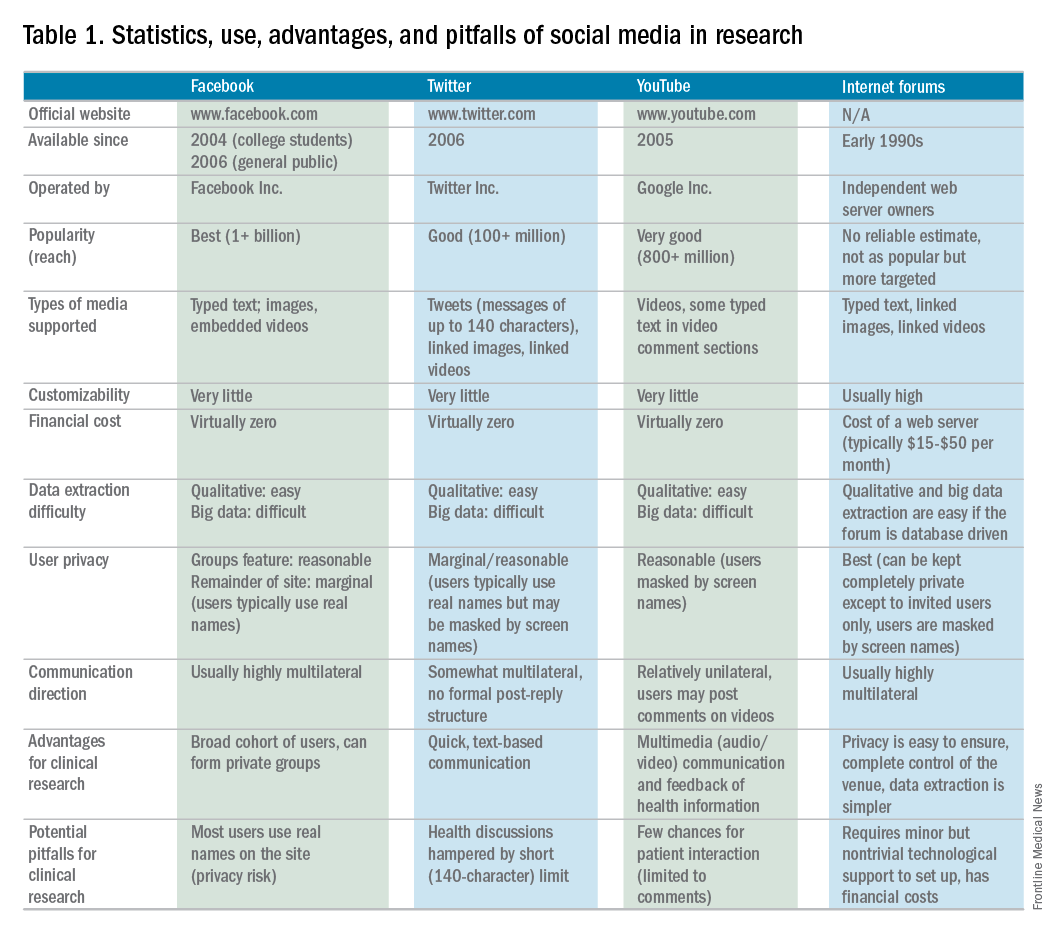
Currently, the use of SM in hepatology research, focused specifically on autoimmune hepatitis (AIH), is under exploration at Indiana University. AIH is a rare autoimmune liver disease that results in immune-mediated destruction of liver cells, possibly resulting in fibrosis, cirrhosis, or liver failure if treatment is unsuccessful. One of the authors (C.L.) used both Facebook and Twitter to construct a large study group of individuals affected with AIH called the Autoimmune Hepatitis Research Network (AHRN; 1,500 members) during the past 2 years.4 Interested individuals have joined this research group after searching for AIH online support groups or reading shared AHRN posts on other media platforms. Between April 2015 and April 2016, there were posts by more than 750 unique active members (more than 50% of the group contributes to discussions), most of whom appear to be either caregivers of AIH patients or AIH patients themselves.
Preliminary informational analysis on this group has shown that C.L. and study collaborators have been able to uncover rich clinical and nonclinical information that otherwise would remain unknown. This research was performed by semi-automated download of the Facebook group’s content and subsequent semantic analysis. Qualitative analysis also was performed by direct reading of patient narratives. Collected clinical information has included histories of medication side effects, familial autoimmune diseases, and comorbid conditions. The most common factors that patients were unlikely to discuss with a provider (e.g., financial issues, employment, personal relationships, use of supplements, and alcohol use) frequently were discussed in the AHRN group, allowing a more transparent view of the complete disease experience.
Beyond research conducted in the current paradigm, the AHRN has provided a rich community construct in which patients offer each other social support. The patient impression of AHRN on Facebook has been overwhelmingly positive, and patients often wonder why such a model has not been used with other diseases. The close digital interaction the author (C.L.) has had with numerous patients and families has promoted other benefits of this methodology: more than 40 new AIH patients from outside Indiana have traveled to Indiana University for medical consultation despite no advertisement.
Conclusions
SM has the potential to transform health care research as a supplement to traditional research methods. Compared with a conventional research model, this methodology has proven to be cost and time effective, wide reaching, and similarly capable of data collection. Use of SM in research has tremendous potential to direct patient-centered research because invested patient collaborators can take an active role in their own disease and may hone investigatory focus on stakeholder priorities. Limitations to this method are known, however; if implemented cautiously, these can be mitigated. Investment in and application of the social mediome by investigators and patients has the potential to support and transform research that otherwise would be impossible.
Acknowledgments
The authors wish to extend their gratitude to the members of the Autoimmune Hepatitis Research Network for their continued proactivity and engagement in autoimmune hepatitis research. Furthermore, the authors are grateful to Dr. Naga Chalasani for his continued mentorship and extensive contributions to the development of social media approaches in clinical investigation.
References
1. Asch, D.A., Rader, D.J., Merchant, R.M. Mining the social mediome. Trends Mol Med. 2015;21:528-9.
2. Brotherton, C.S., Martin, C.A., Long, M.D. et al. Avoidance of fiber is associated with greater risk of Crohn’s disease flare in a 6-month period. Clin Gastroenterol Hepatol. 2016;14:1130-6.
3. Fenner, Y., Garland, S.M., Moore, E.E., et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14:e20.
4. Lammert, C., Comerford, M., Love, J., et al. Investigation gone viral: application of the social mediasphere in research. Gastroenterology. 2015;149:839-43.
5. Wicks, P., Massagli, M., Frost, J., et al. Sharing health data for better outcomes on PatientsLikeMe. J Med Internet Res. 2010;12:e19.
6. Admon, L., Haefner, J.K., Kolenic, G.E., et al. Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016;18:e326.
7. Farnan, J.M., Sulmasy, L.S., Chaudhry, H. Online medical professionalism. Ann Intern Med. 2013;159:158-9.
8. Massachusetts Medical Society: Social Media Guidelines for Physicians. Available from: http://www.massmed.org/Physicians/Legal-and-Regulatory/Social-Media-Guidelines-for-Physicians/#. Accessed: January 3, 2017.
9. Pirraglia, P.A. Kravitz, R.L. Social media: new opportunities, new ethical concerns. J Gen Intern Med. 2013;28:165-6.
10. Duggan, M., Ellison, N.B., Lampe, C. et al. Demographics of key social networking platforms. (Available from:) (Accessed: January 4, 2017) Pew Res Cent Internet Sci Tech. 2015; http://www.pewinternet.org/2015/01/09/demographics-of-key-social-networking-platforms-2
11. Kang, X., Zhao, L., Leung, F., et al. Delivery of Instructions via mobile social media app increases quality of bowel preparation. Clin Gastroenterol Hepatol. 2016;14:429-35.
12. Bajaj, J.S., Heuman, D.M., Sterling, R.K., et al. Validation of EncephalApp, Smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35.
13. Riaz, M.S. Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
14. Zaid, T., Burzawa, J., Basen-Engquist, K., et al. Use of social media to conduct a cross-sectional epidemiologic and quality of life survey of patients with neuroendocrine carcinoma of the cervix: a feasibility study. Gynecol Oncol. 2014;132:149-53.
15. Schumacher, K.R., Stringer, K.A., Donohue, J.E., et al. Social media methods for studying rare diseases. Pediatrics. 2014;133:e1345–53.
Dr. Kulanthaivel and Dr. Jones are in the school of informatics and computing, Purdue University, Indiana University, Indianapolis; Dr. Fogel and Dr. Lammert are in the department of digestive and liver diseases, Indiana University School of Medicine, Indianapolis. This study was supported by KL2TR001106 and UL1TR001108 from the National Institutes of Health, and the Clinical and Translational Sciences Award from the National Center for Advancing Translational Sciences (C.L.). The authors disclose no conflicts.
We are becoming comfortable with the concept of a sharing economy, where resources are shared among many individuals using online forums. Whether activities involve sharing rides (Uber, Lyft, and others), accommodations (Airbnb), or information (social media), underlying attributes include reduced transactional costs, enhanced information transparency, dynamic feedback, and socialization of opportunity. As health care systems realize that they are changing from direct-to-business to a direct-to-customer model, their ability to connect directly with individuals will become a foundational strategy.
This month’s column introduces us to social media as a research tool. Information derived from social media sites can be harvested for critical clinical information (the Centers for Disease and Control and Prevention tracks the spread of influenza using social media analytic tools), research data (patient preferences), and as a recruitment method for clinical studies. Kulanthaivel and colleagues have described their experiences and literature review to help us imagine new ways to collect data at markedly reduced transaction costs (compared to a formal clinical trial). While there are many cautions about the use of social media in your practice or research, we are only beginning to understand its potential.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Medical knowledge, culminating from the collection and translation of patient data, is the primary objective of the clinical research paradigm. The successful conduct of this traditional model has become even more challenging with expansion of costs and a dwindling research infrastructure. Beyond systemic issues, conventional research methods are burdened further by minimal patient engagement, inadequate staffing, and geographic limitations to recruitment. Clinical research also has failed to keep pace with patient demands, and the limited scope of well-funded, disease-specific investigations have left many patients feeling disenfranchised. Social media venues may represent a viable option to surpass these current and evolving barriers when used as an adjunctive approach to traditional clinical investigation.
Advantages and pitfalls in social media research
SM is a new frontier containing a wide spectrum of clinical and qualitative data from connected users (patients). Collection and examination of either individuals’ or groups’ SM information use can provide insight into qualitative life experiences, just as analysis of biologic samples can enable dissection of genetic disease underpinnings. This mediome is analogous to the human genome, both in content and utility.1 Analyzing data streams from SM for interpersonal interactions, message content, and even frequency can provide digital investigators with volumes of information that otherwise would remain unattainable.
Several limitations and potential risks of SM for medical research should be addressed, including the possible compromise of privacy and confidentiality, the use and dissemination of medical advice and information, potential demographic biases, and a required trust of the investigator by patients. Many of these challenges can be similar to traditional methods, however, as in the conventional model, careful management can drastically reduce unwanted study issues.
The risk of Health Insurance Portability and Accountability Act violations must be considered seriously in the context of patient–researcher interactions on SM. Because of the relatively public nature of these venues, patient confidentiality may be at risk if patients choose to divulge personal medical information. However, if proper protective measures are taken to ensure that the venue is secure (e.g., a private or closed group on Facebook or a by-invitation-only online Internet forum), and the researcher vets all patients who request entrance into the group, this risk may be minimized. Moreover, to further reduce any legal liability, the researcher should not provide any medical advice to patients who participate in a SM study. The drive to provide medical direction in study patients with clinical need may be strong because collaborative relationships between investigator and patients are likely to form. Furthermore, digital access to investigators on SM commonly becomes easy for patients. Safe approaches to communication could include redirecting patients to consult with their own doctor for advice, unbiased dissemination of disease-specific educational materials, or depiction of only institutional review board–approved study materials.7,8
The perception that only younger populations use SM may appear to be a significant limitation for its implementation in clinical research. However, this limitation is rapidly becoming less significant because recent studies have shown that the use of SM has become increasingly common among older adults. As of 2014, more than half of the US adult population used Facebook, including 73% and 63% of Internet-using adults ages 30–49 and 50–64 years, respectively.10 SM may not be suitable for all diseases, however, there is likely significant demographic overlap for many disease populations.
Finally, it is imperative for researchers to gain the trust of patients on SM to effectively use these venues for research purposes. Because patient–researcher interaction does not occur face-to-face on these platforms, gaining the trust of patients may be more difficult than it would be in a clinical setting. Thus, patient–patient and patient–researcher communications within SM platforms must be cultivated carefully to instill participant confidence in the research being performed on their behalf. One of the authors (C.L.) has established an SM educational model for this exchange.4 Specifically, he provides patients with a distillation of current field research by posting updates in a research-specific Facebook group and on Twitter. This model not only empowers patients with disease education, it also solidifies the importance of patient investment in disease-specific research. Furthermore, invested patients bring ideas to research, take a more educated and proactive role in their care team, and, ultimately, return to seek more study involvement.
Social media in rare disease research
Rare diseases (conditions with a prevalence of less than 200,000 patients in North America), in particular, are prime for high-yield results and community impact using novel SM approaches. This is the result of established digital support groups, publications with historically low study numbers, and few focused investigators. Several studies of rare diseases have shown considerable advantages of using SM as a study tool. For instance, an existing neuroendocrine cervical cancer Facebook support group recently was used to recruit a geographically widespread cohort of patients with this rare cancer. Through an online survey posted in the Facebook group, patients were able to provide specific information on their treatment, disease, and symptom history, current disease status, and quality of life, including various psychological factors. Without the use of SM, collecting this information would have been virtually impossible because the patients were treated at 51 cancer centers across the country.14

Currently, the use of SM in hepatology research, focused specifically on autoimmune hepatitis (AIH), is under exploration at Indiana University. AIH is a rare autoimmune liver disease that results in immune-mediated destruction of liver cells, possibly resulting in fibrosis, cirrhosis, or liver failure if treatment is unsuccessful. One of the authors (C.L.) used both Facebook and Twitter to construct a large study group of individuals affected with AIH called the Autoimmune Hepatitis Research Network (AHRN; 1,500 members) during the past 2 years.4 Interested individuals have joined this research group after searching for AIH online support groups or reading shared AHRN posts on other media platforms. Between April 2015 and April 2016, there were posts by more than 750 unique active members (more than 50% of the group contributes to discussions), most of whom appear to be either caregivers of AIH patients or AIH patients themselves.
Preliminary informational analysis on this group has shown that C.L. and study collaborators have been able to uncover rich clinical and nonclinical information that otherwise would remain unknown. This research was performed by semi-automated download of the Facebook group’s content and subsequent semantic analysis. Qualitative analysis also was performed by direct reading of patient narratives. Collected clinical information has included histories of medication side effects, familial autoimmune diseases, and comorbid conditions. The most common factors that patients were unlikely to discuss with a provider (e.g., financial issues, employment, personal relationships, use of supplements, and alcohol use) frequently were discussed in the AHRN group, allowing a more transparent view of the complete disease experience.
Beyond research conducted in the current paradigm, the AHRN has provided a rich community construct in which patients offer each other social support. The patient impression of AHRN on Facebook has been overwhelmingly positive, and patients often wonder why such a model has not been used with other diseases. The close digital interaction the author (C.L.) has had with numerous patients and families has promoted other benefits of this methodology: more than 40 new AIH patients from outside Indiana have traveled to Indiana University for medical consultation despite no advertisement.
Conclusions
SM has the potential to transform health care research as a supplement to traditional research methods. Compared with a conventional research model, this methodology has proven to be cost and time effective, wide reaching, and similarly capable of data collection. Use of SM in research has tremendous potential to direct patient-centered research because invested patient collaborators can take an active role in their own disease and may hone investigatory focus on stakeholder priorities. Limitations to this method are known, however; if implemented cautiously, these can be mitigated. Investment in and application of the social mediome by investigators and patients has the potential to support and transform research that otherwise would be impossible.
Acknowledgments
The authors wish to extend their gratitude to the members of the Autoimmune Hepatitis Research Network for their continued proactivity and engagement in autoimmune hepatitis research. Furthermore, the authors are grateful to Dr. Naga Chalasani for his continued mentorship and extensive contributions to the development of social media approaches in clinical investigation.
References
1. Asch, D.A., Rader, D.J., Merchant, R.M. Mining the social mediome. Trends Mol Med. 2015;21:528-9.
2. Brotherton, C.S., Martin, C.A., Long, M.D. et al. Avoidance of fiber is associated with greater risk of Crohn’s disease flare in a 6-month period. Clin Gastroenterol Hepatol. 2016;14:1130-6.
3. Fenner, Y., Garland, S.M., Moore, E.E., et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14:e20.
4. Lammert, C., Comerford, M., Love, J., et al. Investigation gone viral: application of the social mediasphere in research. Gastroenterology. 2015;149:839-43.
5. Wicks, P., Massagli, M., Frost, J., et al. Sharing health data for better outcomes on PatientsLikeMe. J Med Internet Res. 2010;12:e19.
6. Admon, L., Haefner, J.K., Kolenic, G.E., et al. Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016;18:e326.
7. Farnan, J.M., Sulmasy, L.S., Chaudhry, H. Online medical professionalism. Ann Intern Med. 2013;159:158-9.
8. Massachusetts Medical Society: Social Media Guidelines for Physicians. Available from: http://www.massmed.org/Physicians/Legal-and-Regulatory/Social-Media-Guidelines-for-Physicians/#. Accessed: January 3, 2017.
9. Pirraglia, P.A. Kravitz, R.L. Social media: new opportunities, new ethical concerns. J Gen Intern Med. 2013;28:165-6.
10. Duggan, M., Ellison, N.B., Lampe, C. et al. Demographics of key social networking platforms. (Available from:) (Accessed: January 4, 2017) Pew Res Cent Internet Sci Tech. 2015; http://www.pewinternet.org/2015/01/09/demographics-of-key-social-networking-platforms-2
11. Kang, X., Zhao, L., Leung, F., et al. Delivery of Instructions via mobile social media app increases quality of bowel preparation. Clin Gastroenterol Hepatol. 2016;14:429-35.
12. Bajaj, J.S., Heuman, D.M., Sterling, R.K., et al. Validation of EncephalApp, Smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35.
13. Riaz, M.S. Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
14. Zaid, T., Burzawa, J., Basen-Engquist, K., et al. Use of social media to conduct a cross-sectional epidemiologic and quality of life survey of patients with neuroendocrine carcinoma of the cervix: a feasibility study. Gynecol Oncol. 2014;132:149-53.
15. Schumacher, K.R., Stringer, K.A., Donohue, J.E., et al. Social media methods for studying rare diseases. Pediatrics. 2014;133:e1345–53.
Dr. Kulanthaivel and Dr. Jones are in the school of informatics and computing, Purdue University, Indiana University, Indianapolis; Dr. Fogel and Dr. Lammert are in the department of digestive and liver diseases, Indiana University School of Medicine, Indianapolis. This study was supported by KL2TR001106 and UL1TR001108 from the National Institutes of Health, and the Clinical and Translational Sciences Award from the National Center for Advancing Translational Sciences (C.L.). The authors disclose no conflicts.
We are becoming comfortable with the concept of a sharing economy, where resources are shared among many individuals using online forums. Whether activities involve sharing rides (Uber, Lyft, and others), accommodations (Airbnb), or information (social media), underlying attributes include reduced transactional costs, enhanced information transparency, dynamic feedback, and socialization of opportunity. As health care systems realize that they are changing from direct-to-business to a direct-to-customer model, their ability to connect directly with individuals will become a foundational strategy.
This month’s column introduces us to social media as a research tool. Information derived from social media sites can be harvested for critical clinical information (the Centers for Disease and Control and Prevention tracks the spread of influenza using social media analytic tools), research data (patient preferences), and as a recruitment method for clinical studies. Kulanthaivel and colleagues have described their experiences and literature review to help us imagine new ways to collect data at markedly reduced transaction costs (compared to a formal clinical trial). While there are many cautions about the use of social media in your practice or research, we are only beginning to understand its potential.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Medical knowledge, culminating from the collection and translation of patient data, is the primary objective of the clinical research paradigm. The successful conduct of this traditional model has become even more challenging with expansion of costs and a dwindling research infrastructure. Beyond systemic issues, conventional research methods are burdened further by minimal patient engagement, inadequate staffing, and geographic limitations to recruitment. Clinical research also has failed to keep pace with patient demands, and the limited scope of well-funded, disease-specific investigations have left many patients feeling disenfranchised. Social media venues may represent a viable option to surpass these current and evolving barriers when used as an adjunctive approach to traditional clinical investigation.
Advantages and pitfalls in social media research
SM is a new frontier containing a wide spectrum of clinical and qualitative data from connected users (patients). Collection and examination of either individuals’ or groups’ SM information use can provide insight into qualitative life experiences, just as analysis of biologic samples can enable dissection of genetic disease underpinnings. This mediome is analogous to the human genome, both in content and utility.1 Analyzing data streams from SM for interpersonal interactions, message content, and even frequency can provide digital investigators with volumes of information that otherwise would remain unattainable.
Several limitations and potential risks of SM for medical research should be addressed, including the possible compromise of privacy and confidentiality, the use and dissemination of medical advice and information, potential demographic biases, and a required trust of the investigator by patients. Many of these challenges can be similar to traditional methods, however, as in the conventional model, careful management can drastically reduce unwanted study issues.
The risk of Health Insurance Portability and Accountability Act violations must be considered seriously in the context of patient–researcher interactions on SM. Because of the relatively public nature of these venues, patient confidentiality may be at risk if patients choose to divulge personal medical information. However, if proper protective measures are taken to ensure that the venue is secure (e.g., a private or closed group on Facebook or a by-invitation-only online Internet forum), and the researcher vets all patients who request entrance into the group, this risk may be minimized. Moreover, to further reduce any legal liability, the researcher should not provide any medical advice to patients who participate in a SM study. The drive to provide medical direction in study patients with clinical need may be strong because collaborative relationships between investigator and patients are likely to form. Furthermore, digital access to investigators on SM commonly becomes easy for patients. Safe approaches to communication could include redirecting patients to consult with their own doctor for advice, unbiased dissemination of disease-specific educational materials, or depiction of only institutional review board–approved study materials.7,8
The perception that only younger populations use SM may appear to be a significant limitation for its implementation in clinical research. However, this limitation is rapidly becoming less significant because recent studies have shown that the use of SM has become increasingly common among older adults. As of 2014, more than half of the US adult population used Facebook, including 73% and 63% of Internet-using adults ages 30–49 and 50–64 years, respectively.10 SM may not be suitable for all diseases, however, there is likely significant demographic overlap for many disease populations.
Finally, it is imperative for researchers to gain the trust of patients on SM to effectively use these venues for research purposes. Because patient–researcher interaction does not occur face-to-face on these platforms, gaining the trust of patients may be more difficult than it would be in a clinical setting. Thus, patient–patient and patient–researcher communications within SM platforms must be cultivated carefully to instill participant confidence in the research being performed on their behalf. One of the authors (C.L.) has established an SM educational model for this exchange.4 Specifically, he provides patients with a distillation of current field research by posting updates in a research-specific Facebook group and on Twitter. This model not only empowers patients with disease education, it also solidifies the importance of patient investment in disease-specific research. Furthermore, invested patients bring ideas to research, take a more educated and proactive role in their care team, and, ultimately, return to seek more study involvement.
Social media in rare disease research
Rare diseases (conditions with a prevalence of less than 200,000 patients in North America), in particular, are prime for high-yield results and community impact using novel SM approaches. This is the result of established digital support groups, publications with historically low study numbers, and few focused investigators. Several studies of rare diseases have shown considerable advantages of using SM as a study tool. For instance, an existing neuroendocrine cervical cancer Facebook support group recently was used to recruit a geographically widespread cohort of patients with this rare cancer. Through an online survey posted in the Facebook group, patients were able to provide specific information on their treatment, disease, and symptom history, current disease status, and quality of life, including various psychological factors. Without the use of SM, collecting this information would have been virtually impossible because the patients were treated at 51 cancer centers across the country.14

Currently, the use of SM in hepatology research, focused specifically on autoimmune hepatitis (AIH), is under exploration at Indiana University. AIH is a rare autoimmune liver disease that results in immune-mediated destruction of liver cells, possibly resulting in fibrosis, cirrhosis, or liver failure if treatment is unsuccessful. One of the authors (C.L.) used both Facebook and Twitter to construct a large study group of individuals affected with AIH called the Autoimmune Hepatitis Research Network (AHRN; 1,500 members) during the past 2 years.4 Interested individuals have joined this research group after searching for AIH online support groups or reading shared AHRN posts on other media platforms. Between April 2015 and April 2016, there were posts by more than 750 unique active members (more than 50% of the group contributes to discussions), most of whom appear to be either caregivers of AIH patients or AIH patients themselves.
Preliminary informational analysis on this group has shown that C.L. and study collaborators have been able to uncover rich clinical and nonclinical information that otherwise would remain unknown. This research was performed by semi-automated download of the Facebook group’s content and subsequent semantic analysis. Qualitative analysis also was performed by direct reading of patient narratives. Collected clinical information has included histories of medication side effects, familial autoimmune diseases, and comorbid conditions. The most common factors that patients were unlikely to discuss with a provider (e.g., financial issues, employment, personal relationships, use of supplements, and alcohol use) frequently were discussed in the AHRN group, allowing a more transparent view of the complete disease experience.
Beyond research conducted in the current paradigm, the AHRN has provided a rich community construct in which patients offer each other social support. The patient impression of AHRN on Facebook has been overwhelmingly positive, and patients often wonder why such a model has not been used with other diseases. The close digital interaction the author (C.L.) has had with numerous patients and families has promoted other benefits of this methodology: more than 40 new AIH patients from outside Indiana have traveled to Indiana University for medical consultation despite no advertisement.
Conclusions
SM has the potential to transform health care research as a supplement to traditional research methods. Compared with a conventional research model, this methodology has proven to be cost and time effective, wide reaching, and similarly capable of data collection. Use of SM in research has tremendous potential to direct patient-centered research because invested patient collaborators can take an active role in their own disease and may hone investigatory focus on stakeholder priorities. Limitations to this method are known, however; if implemented cautiously, these can be mitigated. Investment in and application of the social mediome by investigators and patients has the potential to support and transform research that otherwise would be impossible.
Acknowledgments
The authors wish to extend their gratitude to the members of the Autoimmune Hepatitis Research Network for their continued proactivity and engagement in autoimmune hepatitis research. Furthermore, the authors are grateful to Dr. Naga Chalasani for his continued mentorship and extensive contributions to the development of social media approaches in clinical investigation.
References
1. Asch, D.A., Rader, D.J., Merchant, R.M. Mining the social mediome. Trends Mol Med. 2015;21:528-9.
2. Brotherton, C.S., Martin, C.A., Long, M.D. et al. Avoidance of fiber is associated with greater risk of Crohn’s disease flare in a 6-month period. Clin Gastroenterol Hepatol. 2016;14:1130-6.
3. Fenner, Y., Garland, S.M., Moore, E.E., et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14:e20.
4. Lammert, C., Comerford, M., Love, J., et al. Investigation gone viral: application of the social mediasphere in research. Gastroenterology. 2015;149:839-43.
5. Wicks, P., Massagli, M., Frost, J., et al. Sharing health data for better outcomes on PatientsLikeMe. J Med Internet Res. 2010;12:e19.
6. Admon, L., Haefner, J.K., Kolenic, G.E., et al. Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016;18:e326.
7. Farnan, J.M., Sulmasy, L.S., Chaudhry, H. Online medical professionalism. Ann Intern Med. 2013;159:158-9.
8. Massachusetts Medical Society: Social Media Guidelines for Physicians. Available from: http://www.massmed.org/Physicians/Legal-and-Regulatory/Social-Media-Guidelines-for-Physicians/#. Accessed: January 3, 2017.
9. Pirraglia, P.A. Kravitz, R.L. Social media: new opportunities, new ethical concerns. J Gen Intern Med. 2013;28:165-6.
10. Duggan, M., Ellison, N.B., Lampe, C. et al. Demographics of key social networking platforms. (Available from:) (Accessed: January 4, 2017) Pew Res Cent Internet Sci Tech. 2015; http://www.pewinternet.org/2015/01/09/demographics-of-key-social-networking-platforms-2
11. Kang, X., Zhao, L., Leung, F., et al. Delivery of Instructions via mobile social media app increases quality of bowel preparation. Clin Gastroenterol Hepatol. 2016;14:429-35.
12. Bajaj, J.S., Heuman, D.M., Sterling, R.K., et al. Validation of EncephalApp, Smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35.
13. Riaz, M.S. Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
14. Zaid, T., Burzawa, J., Basen-Engquist, K., et al. Use of social media to conduct a cross-sectional epidemiologic and quality of life survey of patients with neuroendocrine carcinoma of the cervix: a feasibility study. Gynecol Oncol. 2014;132:149-53.
15. Schumacher, K.R., Stringer, K.A., Donohue, J.E., et al. Social media methods for studying rare diseases. Pediatrics. 2014;133:e1345–53.
Dr. Kulanthaivel and Dr. Jones are in the school of informatics and computing, Purdue University, Indiana University, Indianapolis; Dr. Fogel and Dr. Lammert are in the department of digestive and liver diseases, Indiana University School of Medicine, Indianapolis. This study was supported by KL2TR001106 and UL1TR001108 from the National Institutes of Health, and the Clinical and Translational Sciences Award from the National Center for Advancing Translational Sciences (C.L.). The authors disclose no conflicts.
Challenges facing independent integrated gastroenterology in 2017
The practice of gastroenterology is challenging for community physicians, those employed in multi-specialty clinics or large health care systems and those in academic health centers. Unique challenges confront independent GI practices, and there are mounting regulatory, financial, and operational barriers. Election results of 2016 have thrown us into an even more confusing future. In this month’s Road Ahead column, national GI leaders summarize the major challenges facing independent practices. Each leads (or has led) large GI practices and each has extensive experience with the policies, politics, payers, and pitfalls that impact our specialty. They have written a clear and helpful article for all physicians trying to maintain their independence and patient-focused practices. I have worked in many settings from the VA, to small and then large, independent practice, within a health system and in 2 academic medical centers. There is much to treasure in every type of practice and also many challenges. Physician leaders, both old and young, need to be informed and active in shaping medical policy.
John I. Allen, MD, MBA, AGAF, Editor in Chief
Physicians practicing in independent settings report greater satisfaction with their careers compared with those employed in hospital systems. In a recent survey,1 nearly two-thirds of independent practitioners strongly agreed with the statement, “I like being a physician,” compared with approximately half of those employed by hospital systems. The rapid pace of change in care delivery is forcing all caregivers to modify how they provide care. For physicians practicing in independent settings, understanding, reacting, and adapting to these changes is especially challenging.
It is particularly difficult for physicians and practices to remain abreast and cognizant of the ever-changing rules governing how we deliver care for our patients. The Digestive Health Physicians Association was formed 2 years ago to provide an active voice specifically for independent gastroenterology (GI) practices. The mission of the Digestive Health Physicians Association is to promote and protect the high-quality and cost-efficient care provided in the integrated GI practice model.
In the past decade, meeting the goal of the Triple Aim (improving population health, improving patient experience of care, and reducing the per-capita cost of health care) has become a central tenet of our national health policy strategy, especially since the enactment of the Affordable Care Act. Achieving the goals of the Triple Aim and complying with the changes and new requirements challenges all gastroenterologists, but particularly those working in the independent practice setting, and especially those in small group practices. The Centers for Medicare and Medicaid Services (CMS) recently estimated that under the Merit-Based Incentive Payment System, payment reductions resulting from the first year of reporting in 2017 will occur in 87% of solo practices, in 70% of groups with 2 to 9 physicians, and in 60% of groups with 10 to 24 physicians.2
Preparing yourself and your practice for the changes ahead will require an understanding of the rules, an assessment of your practice’s readiness, and the creation of a plan for compliance to ensure success.
The care model has undergone major changes in the past decade. The development of regional hospital systems has resulted in increasing numbers of employed physicians. Independent gastroenterology practices also have made changes in how they provide care. Vertical integration by independent practices has been a major, positive, and continuing development. As practices have grown more sophisticated with greater areas of specialization, they are increasingly capable of providing services directly to their patients rather than outsourcing them to external providers. Beginning first with endoscopic procedures and now extending to anesthesia, pathology, infusion, and other critical services, increased integration of services across the entire continuum of care has led to improved efficiency and care coordination, benefitting patients with improved outcomes as well as lower costs to our health care system.
The benefits and successes of practice integration, unfortunately, also have made vertically integrated practices a target for regulators and policy makers. Attacks on the integrated delivery model in gastroenterology have at times been supported, if not directly initiated, by our own colleagues in the house of medicine. In this article, we describe some of the threats and challenges confronting independent GI practice.
Anesthesia services
In April 2016, the Florida Society of Anesthesiologists (FSA) made headlines by drawing attention to its role as the relator in a qui tam (whistleblower) lawsuit that it had filed against more than 50 physicians, Ambulatory Surgery Centers, and anesthesia entities. This legal action — which the FSA filed in October 2013 but remained under seal until earlier this year — alleged that the defendants perpetrated Medicare and Medicaid fraud through violations of the federal Anti-Kickback Statute and the False Claims Act. In this lawsuit, the FSA specifically targeted the company model used to provide anesthesia services. Based on publicly available documents, the case currently is in its early stages, although the FSA has made it clear that it views the lawsuit as a blueprint for attacking integrated anesthesia services.
The FSA’s qui tam action in Florida is part of a broader agenda by those who seek to undermine the integrated care model that enables gastroenterologists and other physician specialists to integrate anesthesia services into lawful care models. A website describing the Florida qui tam action hailed the American Society of Anesthesiology for having “repeatedly petitioned the Office of the Inspector General, brought the issue up with Congressional leaders and executive branch regulators, and provided information and legal resources to its members.”3 These efforts to undermine integrated, coordinated care at the federal level also have extended to the state level, in which efforts have been made in front of licensing boards and state legislatures – albeit unsuccessfully – to restrict the integration of anesthesia services.
In-Office Ancillary Services Exception
The In-Office Ancillary Services Exception (IOASE) to the federal physician self-referral statute (the Stark Law), allows physician practices to provide certain services, including diagnostic imaging and anatomic pathology, in an integrated and coordinated fashion within their respective practices when strict criteria are met.
Not surprisingly, competing providers of these services have long fought for the elimination of the IOASE. In 2013, Representative Jackie Speier (D-CA) introduced the Promoting Integrity in Medicare Act. This bill sought to eliminate those legal protections for providing those integrated medical services under the IOASE. Vigorous support for the legislation was provided by a group called the Alliance for Integrity in Medicine, a coalition of organizations including the College of American Pathologists, the American Society for Clinical Pathology, the American Clinical Laboratory Association, and the American College of Radiology. Although that bill did not even receive a vote during the last Congress, Representative Speier has re-introduced it in this current session, and continues to lobby aggressively in support of this legislation. President Obama’s budget for 2016, as the President’s budget proposal had done for the past several years, also included elimination of the IOASE provision. Extensive advocacy efforts by a broad range of specialty organizations have been instrumental to date in defeating this proposal. A study commissioned by the Digestive Health Physicians Association,4 using Medicare data, showed that GI-related anatomic pathology services actually increased more slowly in professional settings (physician offices and laboratories), at an annual rate of 1.2% from 2009 to 2013, compared with the outpatient hospital setting of 3.5% during that same period. Efforts to restrict practice integration similarly are being made at the state level. In California, legislation to eliminate the IOASE under the State’s self-referral law was introduced in 2014. Coordinated efforts by California patient- and physician-interest groups were successful in educating legislators on the value of the integrated care mode and the bill was soundly defeated.
Medicare Part B Drug Benefit
In March 2016, a new threat to integrated care in GI surfaced when the CMS released a proposed rule that would test a new Medicare Part B payment model for infused drugs including infliximab and vedolizumab. Under the proposal, the CMS would reduce the current reimbursement of 6% above average sales price (ASP) to ASP plus 2.5%, plus a flat fee of $16.85 per infusion. CMS calculations in this proposal failed to include the mandatory 2% sequestration of Medicare payments under the Budget Control Act, which means that the actual reimbursement will be less than 1% over ASP. Because many practices are unable to negotiate discounts for these drugs, unintended consequences of this proposal may disrupt care coordination efforts, resulting in movement of infusions performed in the office setting into the more costly hospital setting. Perversely, although the stated intent of this proposal was to reduce incentives for prescribing more expensive drugs, infused biologic agents are actually treatments of last choice for many inflammatory bowel disease patients.
In the absence of less-expensive alternatives, this proposed change in reimbursement likely will reduce access to effective therapy for some of our sickest patients and will not reduce costs. It is hoped that the vigorous advocacy by a coalition of more than 300 medical societies and patient-interest groups along with a majority of members of Congress may result in modifications to this proposal when the final rule is released later this year.
Stark Law
The Stark Law, commonly known as the Physician Self-Referral Law, originally was passed by Congress in 1989 and was substantially amended last in 1993. The Stark Law was enacted to address concerns of potential overuse or inappropriate use of services in a fee-for-service payment system. Health care delivery has changed dramatically since the Stark law was passed 27 years ago, but this statute has not kept pace and is incompatible with new and innovative delivery models that now mandate a shift from fee-for-service payment models to value-based care and the development of risk-sharing arrangements and bundling of services. In 2011, the CMS created a set of waivers for Accountable Care Organizations in the Medicare Shared Savings Program, but these waivers do not apply to many of the alternative payment models under development by independent physicians.
In the past year, Congress repealed the Sustainable Growth Rate formula. Both the Affordable Care Act and the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015 were designed to move our health care system away from fee for service (volume) and toward payment for value. The current Stark Law was created to control arrangements in a fee-for-service system, but the Stark Law now obstructs the ability of physicians in independent practices to coordinate care and work as teams across specialties and with their colleagues who care for patients in other sites of service such as hospitals and academic medical centers.
Congress and CMS recently have heard from dozens of physician organizations, including all of the GI societies, about the need to modernize the Stark Law (by way of updates to the Stark statute and its corresponding regulations) to keep pace with the changes in health care delivery and to ensure successful implementation of the Medicare Access and Children’s Health Insurance Program Reauthorization Act. The changes sought include modifications to the definition of the term group practice to permit coordinated care across specialties and sites of service as well as to promote value-based compensation for all physicians.
Conclusions
To maximally amplify our voices as well our ability to effect positive change, gastroenterologists and other specialists should be actively engaged with the GI societies to help influence those changes proposed. Joining together will facilitate the adaptation by practices to change as it is mandated. The American Gastroenterological Association has long advocated for independent practices promoting optimal patient care delivery. Working cooperatively and collectively with colleagues in all GI professional organizations will enhance our ability to advance the best interests of our patients and our practices.
References
1. Great American Physician Survey 2013. Available from: Physicianspractice.com. Accessed: May 8, 2016.
2. Lowes, R. New Medicare penalty hits small groups, solo physicians hardest. Medscape Medical News. April 28, 2016;
3. The Anesthesia Company Model - FAQs. Available from: http://www.fsahq.org/anesthesia-company-model-faqs. Accessed: May 8, 2016.
4. Milliman White Paper, Medicare anatomic pathology utilization 2009-2013. Available: http://www.dhpassociation.org/wordpress/wp-content/uploads/2015/07/milliman-03-2009-2013-medicare-utilization-analysis.pdf. Accessed: May 8, 2016.
Dr. Rosenberg is a board-certified gastroenterologist who is currently the president of Illinois Gastroenterology Group, Highland Park, Ill; Dr Kim is a gastroenterologist at South Denver Gastroenterology, P.C., Lone Tree, Colo.; and Dr. Ketover is a gastroenterologist and is president and CEO of Minnesota Gastroenterology, P.A.; St. Paul. The authors disclose no conflicts.
The practice of gastroenterology is challenging for community physicians, those employed in multi-specialty clinics or large health care systems and those in academic health centers. Unique challenges confront independent GI practices, and there are mounting regulatory, financial, and operational barriers. Election results of 2016 have thrown us into an even more confusing future. In this month’s Road Ahead column, national GI leaders summarize the major challenges facing independent practices. Each leads (or has led) large GI practices and each has extensive experience with the policies, politics, payers, and pitfalls that impact our specialty. They have written a clear and helpful article for all physicians trying to maintain their independence and patient-focused practices. I have worked in many settings from the VA, to small and then large, independent practice, within a health system and in 2 academic medical centers. There is much to treasure in every type of practice and also many challenges. Physician leaders, both old and young, need to be informed and active in shaping medical policy.
John I. Allen, MD, MBA, AGAF, Editor in Chief
Physicians practicing in independent settings report greater satisfaction with their careers compared with those employed in hospital systems. In a recent survey,1 nearly two-thirds of independent practitioners strongly agreed with the statement, “I like being a physician,” compared with approximately half of those employed by hospital systems. The rapid pace of change in care delivery is forcing all caregivers to modify how they provide care. For physicians practicing in independent settings, understanding, reacting, and adapting to these changes is especially challenging.
It is particularly difficult for physicians and practices to remain abreast and cognizant of the ever-changing rules governing how we deliver care for our patients. The Digestive Health Physicians Association was formed 2 years ago to provide an active voice specifically for independent gastroenterology (GI) practices. The mission of the Digestive Health Physicians Association is to promote and protect the high-quality and cost-efficient care provided in the integrated GI practice model.
In the past decade, meeting the goal of the Triple Aim (improving population health, improving patient experience of care, and reducing the per-capita cost of health care) has become a central tenet of our national health policy strategy, especially since the enactment of the Affordable Care Act. Achieving the goals of the Triple Aim and complying with the changes and new requirements challenges all gastroenterologists, but particularly those working in the independent practice setting, and especially those in small group practices. The Centers for Medicare and Medicaid Services (CMS) recently estimated that under the Merit-Based Incentive Payment System, payment reductions resulting from the first year of reporting in 2017 will occur in 87% of solo practices, in 70% of groups with 2 to 9 physicians, and in 60% of groups with 10 to 24 physicians.2
Preparing yourself and your practice for the changes ahead will require an understanding of the rules, an assessment of your practice’s readiness, and the creation of a plan for compliance to ensure success.
The care model has undergone major changes in the past decade. The development of regional hospital systems has resulted in increasing numbers of employed physicians. Independent gastroenterology practices also have made changes in how they provide care. Vertical integration by independent practices has been a major, positive, and continuing development. As practices have grown more sophisticated with greater areas of specialization, they are increasingly capable of providing services directly to their patients rather than outsourcing them to external providers. Beginning first with endoscopic procedures and now extending to anesthesia, pathology, infusion, and other critical services, increased integration of services across the entire continuum of care has led to improved efficiency and care coordination, benefitting patients with improved outcomes as well as lower costs to our health care system.
The benefits and successes of practice integration, unfortunately, also have made vertically integrated practices a target for regulators and policy makers. Attacks on the integrated delivery model in gastroenterology have at times been supported, if not directly initiated, by our own colleagues in the house of medicine. In this article, we describe some of the threats and challenges confronting independent GI practice.
Anesthesia services
In April 2016, the Florida Society of Anesthesiologists (FSA) made headlines by drawing attention to its role as the relator in a qui tam (whistleblower) lawsuit that it had filed against more than 50 physicians, Ambulatory Surgery Centers, and anesthesia entities. This legal action — which the FSA filed in October 2013 but remained under seal until earlier this year — alleged that the defendants perpetrated Medicare and Medicaid fraud through violations of the federal Anti-Kickback Statute and the False Claims Act. In this lawsuit, the FSA specifically targeted the company model used to provide anesthesia services. Based on publicly available documents, the case currently is in its early stages, although the FSA has made it clear that it views the lawsuit as a blueprint for attacking integrated anesthesia services.
The FSA’s qui tam action in Florida is part of a broader agenda by those who seek to undermine the integrated care model that enables gastroenterologists and other physician specialists to integrate anesthesia services into lawful care models. A website describing the Florida qui tam action hailed the American Society of Anesthesiology for having “repeatedly petitioned the Office of the Inspector General, brought the issue up with Congressional leaders and executive branch regulators, and provided information and legal resources to its members.”3 These efforts to undermine integrated, coordinated care at the federal level also have extended to the state level, in which efforts have been made in front of licensing boards and state legislatures – albeit unsuccessfully – to restrict the integration of anesthesia services.
In-Office Ancillary Services Exception
The In-Office Ancillary Services Exception (IOASE) to the federal physician self-referral statute (the Stark Law), allows physician practices to provide certain services, including diagnostic imaging and anatomic pathology, in an integrated and coordinated fashion within their respective practices when strict criteria are met.
Not surprisingly, competing providers of these services have long fought for the elimination of the IOASE. In 2013, Representative Jackie Speier (D-CA) introduced the Promoting Integrity in Medicare Act. This bill sought to eliminate those legal protections for providing those integrated medical services under the IOASE. Vigorous support for the legislation was provided by a group called the Alliance for Integrity in Medicine, a coalition of organizations including the College of American Pathologists, the American Society for Clinical Pathology, the American Clinical Laboratory Association, and the American College of Radiology. Although that bill did not even receive a vote during the last Congress, Representative Speier has re-introduced it in this current session, and continues to lobby aggressively in support of this legislation. President Obama’s budget for 2016, as the President’s budget proposal had done for the past several years, also included elimination of the IOASE provision. Extensive advocacy efforts by a broad range of specialty organizations have been instrumental to date in defeating this proposal. A study commissioned by the Digestive Health Physicians Association,4 using Medicare data, showed that GI-related anatomic pathology services actually increased more slowly in professional settings (physician offices and laboratories), at an annual rate of 1.2% from 2009 to 2013, compared with the outpatient hospital setting of 3.5% during that same period. Efforts to restrict practice integration similarly are being made at the state level. In California, legislation to eliminate the IOASE under the State’s self-referral law was introduced in 2014. Coordinated efforts by California patient- and physician-interest groups were successful in educating legislators on the value of the integrated care mode and the bill was soundly defeated.
Medicare Part B Drug Benefit
In March 2016, a new threat to integrated care in GI surfaced when the CMS released a proposed rule that would test a new Medicare Part B payment model for infused drugs including infliximab and vedolizumab. Under the proposal, the CMS would reduce the current reimbursement of 6% above average sales price (ASP) to ASP plus 2.5%, plus a flat fee of $16.85 per infusion. CMS calculations in this proposal failed to include the mandatory 2% sequestration of Medicare payments under the Budget Control Act, which means that the actual reimbursement will be less than 1% over ASP. Because many practices are unable to negotiate discounts for these drugs, unintended consequences of this proposal may disrupt care coordination efforts, resulting in movement of infusions performed in the office setting into the more costly hospital setting. Perversely, although the stated intent of this proposal was to reduce incentives for prescribing more expensive drugs, infused biologic agents are actually treatments of last choice for many inflammatory bowel disease patients.
In the absence of less-expensive alternatives, this proposed change in reimbursement likely will reduce access to effective therapy for some of our sickest patients and will not reduce costs. It is hoped that the vigorous advocacy by a coalition of more than 300 medical societies and patient-interest groups along with a majority of members of Congress may result in modifications to this proposal when the final rule is released later this year.
Stark Law
The Stark Law, commonly known as the Physician Self-Referral Law, originally was passed by Congress in 1989 and was substantially amended last in 1993. The Stark Law was enacted to address concerns of potential overuse or inappropriate use of services in a fee-for-service payment system. Health care delivery has changed dramatically since the Stark law was passed 27 years ago, but this statute has not kept pace and is incompatible with new and innovative delivery models that now mandate a shift from fee-for-service payment models to value-based care and the development of risk-sharing arrangements and bundling of services. In 2011, the CMS created a set of waivers for Accountable Care Organizations in the Medicare Shared Savings Program, but these waivers do not apply to many of the alternative payment models under development by independent physicians.
In the past year, Congress repealed the Sustainable Growth Rate formula. Both the Affordable Care Act and the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015 were designed to move our health care system away from fee for service (volume) and toward payment for value. The current Stark Law was created to control arrangements in a fee-for-service system, but the Stark Law now obstructs the ability of physicians in independent practices to coordinate care and work as teams across specialties and with their colleagues who care for patients in other sites of service such as hospitals and academic medical centers.
Congress and CMS recently have heard from dozens of physician organizations, including all of the GI societies, about the need to modernize the Stark Law (by way of updates to the Stark statute and its corresponding regulations) to keep pace with the changes in health care delivery and to ensure successful implementation of the Medicare Access and Children’s Health Insurance Program Reauthorization Act. The changes sought include modifications to the definition of the term group practice to permit coordinated care across specialties and sites of service as well as to promote value-based compensation for all physicians.
Conclusions
To maximally amplify our voices as well our ability to effect positive change, gastroenterologists and other specialists should be actively engaged with the GI societies to help influence those changes proposed. Joining together will facilitate the adaptation by practices to change as it is mandated. The American Gastroenterological Association has long advocated for independent practices promoting optimal patient care delivery. Working cooperatively and collectively with colleagues in all GI professional organizations will enhance our ability to advance the best interests of our patients and our practices.
References
1. Great American Physician Survey 2013. Available from: Physicianspractice.com. Accessed: May 8, 2016.
2. Lowes, R. New Medicare penalty hits small groups, solo physicians hardest. Medscape Medical News. April 28, 2016;
3. The Anesthesia Company Model - FAQs. Available from: http://www.fsahq.org/anesthesia-company-model-faqs. Accessed: May 8, 2016.
4. Milliman White Paper, Medicare anatomic pathology utilization 2009-2013. Available: http://www.dhpassociation.org/wordpress/wp-content/uploads/2015/07/milliman-03-2009-2013-medicare-utilization-analysis.pdf. Accessed: May 8, 2016.
Dr. Rosenberg is a board-certified gastroenterologist who is currently the president of Illinois Gastroenterology Group, Highland Park, Ill; Dr Kim is a gastroenterologist at South Denver Gastroenterology, P.C., Lone Tree, Colo.; and Dr. Ketover is a gastroenterologist and is president and CEO of Minnesota Gastroenterology, P.A.; St. Paul. The authors disclose no conflicts.
The practice of gastroenterology is challenging for community physicians, those employed in multi-specialty clinics or large health care systems and those in academic health centers. Unique challenges confront independent GI practices, and there are mounting regulatory, financial, and operational barriers. Election results of 2016 have thrown us into an even more confusing future. In this month’s Road Ahead column, national GI leaders summarize the major challenges facing independent practices. Each leads (or has led) large GI practices and each has extensive experience with the policies, politics, payers, and pitfalls that impact our specialty. They have written a clear and helpful article for all physicians trying to maintain their independence and patient-focused practices. I have worked in many settings from the VA, to small and then large, independent practice, within a health system and in 2 academic medical centers. There is much to treasure in every type of practice and also many challenges. Physician leaders, both old and young, need to be informed and active in shaping medical policy.
John I. Allen, MD, MBA, AGAF, Editor in Chief
Physicians practicing in independent settings report greater satisfaction with their careers compared with those employed in hospital systems. In a recent survey,1 nearly two-thirds of independent practitioners strongly agreed with the statement, “I like being a physician,” compared with approximately half of those employed by hospital systems. The rapid pace of change in care delivery is forcing all caregivers to modify how they provide care. For physicians practicing in independent settings, understanding, reacting, and adapting to these changes is especially challenging.
It is particularly difficult for physicians and practices to remain abreast and cognizant of the ever-changing rules governing how we deliver care for our patients. The Digestive Health Physicians Association was formed 2 years ago to provide an active voice specifically for independent gastroenterology (GI) practices. The mission of the Digestive Health Physicians Association is to promote and protect the high-quality and cost-efficient care provided in the integrated GI practice model.
In the past decade, meeting the goal of the Triple Aim (improving population health, improving patient experience of care, and reducing the per-capita cost of health care) has become a central tenet of our national health policy strategy, especially since the enactment of the Affordable Care Act. Achieving the goals of the Triple Aim and complying with the changes and new requirements challenges all gastroenterologists, but particularly those working in the independent practice setting, and especially those in small group practices. The Centers for Medicare and Medicaid Services (CMS) recently estimated that under the Merit-Based Incentive Payment System, payment reductions resulting from the first year of reporting in 2017 will occur in 87% of solo practices, in 70% of groups with 2 to 9 physicians, and in 60% of groups with 10 to 24 physicians.2
Preparing yourself and your practice for the changes ahead will require an understanding of the rules, an assessment of your practice’s readiness, and the creation of a plan for compliance to ensure success.
The care model has undergone major changes in the past decade. The development of regional hospital systems has resulted in increasing numbers of employed physicians. Independent gastroenterology practices also have made changes in how they provide care. Vertical integration by independent practices has been a major, positive, and continuing development. As practices have grown more sophisticated with greater areas of specialization, they are increasingly capable of providing services directly to their patients rather than outsourcing them to external providers. Beginning first with endoscopic procedures and now extending to anesthesia, pathology, infusion, and other critical services, increased integration of services across the entire continuum of care has led to improved efficiency and care coordination, benefitting patients with improved outcomes as well as lower costs to our health care system.
The benefits and successes of practice integration, unfortunately, also have made vertically integrated practices a target for regulators and policy makers. Attacks on the integrated delivery model in gastroenterology have at times been supported, if not directly initiated, by our own colleagues in the house of medicine. In this article, we describe some of the threats and challenges confronting independent GI practice.
Anesthesia services
In April 2016, the Florida Society of Anesthesiologists (FSA) made headlines by drawing attention to its role as the relator in a qui tam (whistleblower) lawsuit that it had filed against more than 50 physicians, Ambulatory Surgery Centers, and anesthesia entities. This legal action — which the FSA filed in October 2013 but remained under seal until earlier this year — alleged that the defendants perpetrated Medicare and Medicaid fraud through violations of the federal Anti-Kickback Statute and the False Claims Act. In this lawsuit, the FSA specifically targeted the company model used to provide anesthesia services. Based on publicly available documents, the case currently is in its early stages, although the FSA has made it clear that it views the lawsuit as a blueprint for attacking integrated anesthesia services.
The FSA’s qui tam action in Florida is part of a broader agenda by those who seek to undermine the integrated care model that enables gastroenterologists and other physician specialists to integrate anesthesia services into lawful care models. A website describing the Florida qui tam action hailed the American Society of Anesthesiology for having “repeatedly petitioned the Office of the Inspector General, brought the issue up with Congressional leaders and executive branch regulators, and provided information and legal resources to its members.”3 These efforts to undermine integrated, coordinated care at the federal level also have extended to the state level, in which efforts have been made in front of licensing boards and state legislatures – albeit unsuccessfully – to restrict the integration of anesthesia services.
In-Office Ancillary Services Exception
The In-Office Ancillary Services Exception (IOASE) to the federal physician self-referral statute (the Stark Law), allows physician practices to provide certain services, including diagnostic imaging and anatomic pathology, in an integrated and coordinated fashion within their respective practices when strict criteria are met.
Not surprisingly, competing providers of these services have long fought for the elimination of the IOASE. In 2013, Representative Jackie Speier (D-CA) introduced the Promoting Integrity in Medicare Act. This bill sought to eliminate those legal protections for providing those integrated medical services under the IOASE. Vigorous support for the legislation was provided by a group called the Alliance for Integrity in Medicine, a coalition of organizations including the College of American Pathologists, the American Society for Clinical Pathology, the American Clinical Laboratory Association, and the American College of Radiology. Although that bill did not even receive a vote during the last Congress, Representative Speier has re-introduced it in this current session, and continues to lobby aggressively in support of this legislation. President Obama’s budget for 2016, as the President’s budget proposal had done for the past several years, also included elimination of the IOASE provision. Extensive advocacy efforts by a broad range of specialty organizations have been instrumental to date in defeating this proposal. A study commissioned by the Digestive Health Physicians Association,4 using Medicare data, showed that GI-related anatomic pathology services actually increased more slowly in professional settings (physician offices and laboratories), at an annual rate of 1.2% from 2009 to 2013, compared with the outpatient hospital setting of 3.5% during that same period. Efforts to restrict practice integration similarly are being made at the state level. In California, legislation to eliminate the IOASE under the State’s self-referral law was introduced in 2014. Coordinated efforts by California patient- and physician-interest groups were successful in educating legislators on the value of the integrated care mode and the bill was soundly defeated.
Medicare Part B Drug Benefit
In March 2016, a new threat to integrated care in GI surfaced when the CMS released a proposed rule that would test a new Medicare Part B payment model for infused drugs including infliximab and vedolizumab. Under the proposal, the CMS would reduce the current reimbursement of 6% above average sales price (ASP) to ASP plus 2.5%, plus a flat fee of $16.85 per infusion. CMS calculations in this proposal failed to include the mandatory 2% sequestration of Medicare payments under the Budget Control Act, which means that the actual reimbursement will be less than 1% over ASP. Because many practices are unable to negotiate discounts for these drugs, unintended consequences of this proposal may disrupt care coordination efforts, resulting in movement of infusions performed in the office setting into the more costly hospital setting. Perversely, although the stated intent of this proposal was to reduce incentives for prescribing more expensive drugs, infused biologic agents are actually treatments of last choice for many inflammatory bowel disease patients.
In the absence of less-expensive alternatives, this proposed change in reimbursement likely will reduce access to effective therapy for some of our sickest patients and will not reduce costs. It is hoped that the vigorous advocacy by a coalition of more than 300 medical societies and patient-interest groups along with a majority of members of Congress may result in modifications to this proposal when the final rule is released later this year.
Stark Law
The Stark Law, commonly known as the Physician Self-Referral Law, originally was passed by Congress in 1989 and was substantially amended last in 1993. The Stark Law was enacted to address concerns of potential overuse or inappropriate use of services in a fee-for-service payment system. Health care delivery has changed dramatically since the Stark law was passed 27 years ago, but this statute has not kept pace and is incompatible with new and innovative delivery models that now mandate a shift from fee-for-service payment models to value-based care and the development of risk-sharing arrangements and bundling of services. In 2011, the CMS created a set of waivers for Accountable Care Organizations in the Medicare Shared Savings Program, but these waivers do not apply to many of the alternative payment models under development by independent physicians.
In the past year, Congress repealed the Sustainable Growth Rate formula. Both the Affordable Care Act and the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015 were designed to move our health care system away from fee for service (volume) and toward payment for value. The current Stark Law was created to control arrangements in a fee-for-service system, but the Stark Law now obstructs the ability of physicians in independent practices to coordinate care and work as teams across specialties and with their colleagues who care for patients in other sites of service such as hospitals and academic medical centers.
Congress and CMS recently have heard from dozens of physician organizations, including all of the GI societies, about the need to modernize the Stark Law (by way of updates to the Stark statute and its corresponding regulations) to keep pace with the changes in health care delivery and to ensure successful implementation of the Medicare Access and Children’s Health Insurance Program Reauthorization Act. The changes sought include modifications to the definition of the term group practice to permit coordinated care across specialties and sites of service as well as to promote value-based compensation for all physicians.
Conclusions
To maximally amplify our voices as well our ability to effect positive change, gastroenterologists and other specialists should be actively engaged with the GI societies to help influence those changes proposed. Joining together will facilitate the adaptation by practices to change as it is mandated. The American Gastroenterological Association has long advocated for independent practices promoting optimal patient care delivery. Working cooperatively and collectively with colleagues in all GI professional organizations will enhance our ability to advance the best interests of our patients and our practices.
References
1. Great American Physician Survey 2013. Available from: Physicianspractice.com. Accessed: May 8, 2016.
2. Lowes, R. New Medicare penalty hits small groups, solo physicians hardest. Medscape Medical News. April 28, 2016;
3. The Anesthesia Company Model - FAQs. Available from: http://www.fsahq.org/anesthesia-company-model-faqs. Accessed: May 8, 2016.
4. Milliman White Paper, Medicare anatomic pathology utilization 2009-2013. Available: http://www.dhpassociation.org/wordpress/wp-content/uploads/2015/07/milliman-03-2009-2013-medicare-utilization-analysis.pdf. Accessed: May 8, 2016.
Dr. Rosenberg is a board-certified gastroenterologist who is currently the president of Illinois Gastroenterology Group, Highland Park, Ill; Dr Kim is a gastroenterologist at South Denver Gastroenterology, P.C., Lone Tree, Colo.; and Dr. Ketover is a gastroenterologist and is president and CEO of Minnesota Gastroenterology, P.A.; St. Paul. The authors disclose no conflicts.
Integration of telemedicine into clinical gastroenterology and hepatology practice
Two trends in health care delivery that will continue unabated are a) reimbursement pressure and b) increasing demand for our services. Practices that care for patients with complex and chronic conditions are exploring innovative means to expand their care footprint in an economically viable way. One approach currently being used by many health systems is telemedicine. Telemedicine is care delivered remotely using some type of electronic communication. Potentially, telemedicine will allow us to provide specialty services remotely to primary care physicians or even patients. The University of Michigan inflammatory bowel disease program is piloting remote video conferencing, integrated within the electronic medical record system, to provide specialty gastrointestinal consultation directly to Crohn’s and ulcerative colitis patients within their homes. The University of Michigan Health System has an office ready to arrange rapid teleconsultation for any provider. Payment for services has been secured from several payers after health system negotiations. This practice is well established in multiple specialties and settings. In this month’s column, two telemedicine experts review the state of the field, so you too can participate. This innovation is something you should consider for your practice. Technology and payment mechanisms are now available.
John I. Allen, MD, MBA, AGAF
Editor in Chief
As defined by the American Telemedicine Association (ATA), telemedicine is the exchange of medical information from one site to another via electronic communication to improve a patient’s clinical health status.1 If we include care provided over the telephone via providers and nurses between office visits, telemedicine has been practiced for decades. A recent study from the University of Pittsburgh documented 32,667 phone calls from 3,118 patients with inflammatory bowel disease (IBD) in 2010. Seventy-five percent of these calls were related to patient concerns or were generated by the nurse because of changes in the treatment plan.2 If these results are applied to a representative work week, busy IBD centers typically handle more than 100 phone calls per day.3 Telemedicine in clinical practice has expanded to include a variety of modalities such as two-way video, email, or secure messaging through electronic medical records systems, smartphones, wireless tools, and other forms of telecommunication technology (see Figure 1). The increase in use of telemedicine in practice has been driven by a number of factors.
First, it is almost universal that patients have access to a computer and/or cellular telephone. According to the Pew Research Center’s Internet and American Life Project, as of May 2013, 91% of adults are using cellphones.4 As patients have become more connected digitally, it is natural that they desire delivery of services, including health care services, electronically. Second, despite advances in medical, endoscopic, and surgical treatment, many patients still have suboptimal outcomes. There are many reasons for this, including but not limited to nonadherence, poor patient education, inadequate monitoring of symptoms and side effects, concurrent psychiatric disease, comorbid medical conditions, low self-efficacy, and limited access to health care; these issues can be addressed, at least in part, by telemedicine. Finally, patients are also seeking more efficient and convenient ways to receive their care,; including travel and wait times, an average office visit takes up to 2 hours.5
Enhanced monitoring and self-care through use of telemedicine technologies
Several groups have implemented telemedicine to improve monitoring and self-care in patients with IBD. Our group at the University of Maryland, Baltimore, has developed several systems to improve care as part of research protocols. Our first telemedicine system included a laptop computer and electronic weight scale connected telephonically to a server. Patients were asked questions about bowel symptoms, medication use, side effects, and body weight measurements. They also received educational messages. This system, IBD Home Automated Telemanagement (HAT), required installation in the patient’s home by a technical team. Our preliminary results demonstrated that patients were very receptive of the technology.6 In a small pilot study (n = 34), we demonstrated that 88% of patients were adherent to self-assessment during a period of 6 months. In addition, patients experienced a reduction in disease activity, improved quality of life, and increased disease state awareness.7 In a small, randomized, controlled follow-up trial, we demonstrated that use of an ulcerative colitis (UC) telemanagement system (UC HAT) resulted in improved quality of life and decreased disease activity from baseline during 1 year compared with controls. The UC HAT system was enhanced to include self-care plans that were based on patient reporting of symptoms. Fewer participants completed the study in the UC HAT, compared with the control, group (56% vs 72%).8 We theorized that participant dropout was higher in the UC HAT group because of the requirement for a technician to visit the home to install or service the system. Hence, as part of a randomized controlled trial, our group has collaborated with the University of Pittsburgh and Vanderbilt University to assess a new telemedicine system that monitors patients by using text messaging.9 Three hundred forty-eight patients were recruited for this ongoing clinical trial. Thus far, 83%–84% of participants in the intervention arms have completed the 1-year study.
Elkjaer et al. evaluated the impact of a web-based treatment program and patient education center in a convenience sample of patients with UC.10 All 21 patients reported the ability to initiate a self-care plan. Furthermore, participants experienced improvements in knowledge after interaction with the patient education center.10 The web-based self-management and treatment approach was compared with standard of care in 333 patients with mild-to-moderate UC from Ireland and Denmark.11 Only 135 patients (41%) completed the 1-year study. Web subjects were more adherent with acute treatment, demonstrated improved disease knowledge and quality of life, experienced shorter relapses, and had fewer office and urgent care visits. Conversely, web group patients generated more emails and telephone calls. In the Irish arm, the results were similar; however, there was no difference in quality of life between groups, and the relapse rate was higher in the web group, compared with controls.11 A 2012 study by the same group investigated the efficacy of web-based monitoring of Crohn’s disease activity for individualized dosing of infliximab maintenance therapy. Twenty-seven patients were enrolled; 17 completed 52 weeks and 6 completed 26 weeks of follow-up. Patients recorded their symptoms weekly via a web-based portal; on the basis of symptom scores, patients were instructed to contact their physician for an infliximab infusion. Fifty percent of the patients were able to tolerate intervals greater than 8 weeks, whereas 36% required shorter intervals.12 This concept of web-based personalized treatment was further investigated in a 2014 study evaluating 86 patients with mild-to-moderate UC. Mesalamine treatment was individualized on the basis of a composite index of clinical symptoms and fecal calprotectin levels. Use of the web application was associated with decreased disease activity scores and lower fecal calprotectin levels despite dose reduction in 88% of patients at week 12.13
The eIBD program developed at the University of California, Los Angeles, also uses a web-based platform to monitor patients. After an initial training session with an IBD nurse specialist, patients are able to view clinical results and view and update their disease activity status, quality of life, and work productivity remotely. Patients interact with the eIBD program by using a tablet or home computer. Self-monitoring was found to correlate well with an in-person assessment of symptoms and disease activity.14 Patient care is organized into evidence-based pathways on the basis of disease status and the medication regimen. Each pathway has a defined number of clinic and electronic visits and laboratory sets. Patients can also access support programs such as My Academy, My Work, My Coach, My Physical Fitness, and My Diet. When University of California, Los Angeles, IBD patients were compared with matched controls by using an administrative claims database, they were significantly less likely to use steroids and had fewer hospitalizations and emergency department visits.15
HealthPROMISE is an application developed at Mount Sinai to collect patient-reported outcomes and to provide decision support. Patient-reported symptoms and quality of life are integrated into the electronic medical records system; providers can view the information in real time to better manage their panel of patients. HealthPROMISE is currently being evaluated as part of a pragmatic, multicenter, randomized controlled trial.16 EncephalApp is a mobile phone application used to assess patients for hepatic encephalopathy. The application was tested in 167 cirrhotic patients, 38% of whom had overt encephalopathy, and 114 controls. The application was shown to have excellent discriminant ability to detect encephalopathy, and importantly, EnecphalApp times correlated with motor vehicle accidents and illegal turns in a driving simulation test.17 A number of other mobile applications have been developed to support patients with chronic illnesses. These applications can be integrated with wearable devices, and some have been approved by the Food and Drug Administration.18
Telehealth and teleconsultation
Advancements in telemedicine have outpaced the ability of legislators and institutional officials to provide oversight on legal and regulatory issues. Each state sets requirements for providers to engage in telehealth activities. The ATA published the State Telemedicine Gap Analysis to address specific requirements and limitations for each state.19 Issues addressed in this document include the requirement for a face-to-face visit before a telehealth visit, informed consent, and interstate practice. Virtually all states have barriers to providing telemedicine services unless the provider is licensed in the state where the patient resides. To promote telemedicine, the Federation of State Medical Boards proposed the development of an Interstate Licensure Compact in which 17 states participate (AL, AZ, CO, ID, IL, IA, KS, MN, MS, MT, NV, NH, SD, UT, WV, WI, and WY).20 Two key principles include defining the practice of medicine as the location in which the patient resides and placing the provider under the jurisdiction of the state in which the practice occurs. The TELE-MED Act of 2015 has proposed allowing Medicare physicians to provide telehealth services to patients regardless of the state in which they reside.21 In regard to liability, the number of malpractice cases involving telemedicine services is low; most are related to e-prescribing, as opposed to care provided during teleconsultation services.22
However, some unique liability issues relative to telehealth encounters exist. First, when considering standard of care, what do you compare a telemedicine encounter with? Hardware or software malfunctions can occur, with a subsequent inability to provide the telemedicine service. Loss of protected health information through hackers or equipment failure is another potential threat. Reimbursement for telehealth services is also regulated by states and is subject to wide variability; 29 states have laws in place requiring private payers to reimburse for telehealth services at the same level as an in-person encounter.19 The ATA recently published an analysis of issues related to reimbursements.23 In addition to parity, key issues that must be addressed include the failure of the majority of state health plans to provide coverage for telehealth services to employees, restrictions on providing telehealth in nonrural settings, restrictions that are based on the type of health care provider, and restrictions on home monitoring.
The Mayo Clinic, Rochester, Minn., offers outreach to its health system affiliates via a secure video conferencing platform to allow face-to-face consultations for patients with IBD. Consultative appointments are scheduled during preassigned blocks of outreach time on the clinician’s calendar. A nurse transcribes any recommendation that requires an order from the referring gastroenterologist. Health care providers offering video consultation are required to have a medical license for the state in which the patient resides and to be credentialed by the facility to which the Mayo Clinic provides services and the payer reimbursing for the service. The majority of consultations are for discussions regarding medical management: when to start a biologic, safety concerns, monitoring strategies, or for a second opinion regarding the need for surgery for refractory UC or fibrostenotic Crohn’s disease. Access to imaging and laboratory work is facilitated through previsit evaluation performed by a nurse in the referring practice.
If services are provided via consultation to a patient at a non–Mayo Clinic facility via the Affiliated Care Network, there is a legal contract outlining reimbursement as well as terms and conditions. For asynchronous consultation where there is interaction with a provider but the patient is not directly involved (no face-to-face consultation), the practice of medicine regulations vary from state to state as outlined above. Credentialing is not required for provider-to-provider consultation at each site but sometimes a license is. However, for all states, electronic health record documentation of the clinical question and recommendations is important. Substantial administrative infrastructure is required to manage the quality of e-consult responses so that they are timely and the clinical notes meet the needs of the requesting provider. This is supported by a secure online portal that exchanges electronic health record information and the clinical note generated. Mayo Clinic providers are licensed for multiple states to provide medical consultations to various affiliated hospitals throughout the United States. The consulting physician always has the option to recommend a full face-to-face consultation if review of the records provided indicates the patient appears to be too complicated. The telehealth efforts at the Mayo Clinic are not isolated to gastroenterology; it is estimated that, through expanded use of telehealth, Mayo Clinic will provide care nationally and internationally for 200 million people by 2020.24
From April 2015 to May 2016, at the University of Maryland, Baltimore, we conducted 89 telehealth visits. According to state regulations and payer restrictions on reimbursement, patients were eligible to undergo telehealth visits if they had a prior face-to-face visit and were insured by Blue Cross Blue Shield. Eligible patients provided informed consent to participate in the telehealth visit. Patients received an email with instructions on how to download the required software (VidyoDesktop version 3.0.4[001]; Vidyo, Hackensack, N.J.) onto the patient’s home computer, tablet, or smartphone. An office assistant conducted a test visit to make sure that the connection was adequate. Eighty-three percent of patients reported that using the system was not complicated at all or only slightly complicated. Seventy-one percent reported that the telehealth visit took significantly less or less time than a routine encounter; 88% said that all their concerns were addressed during the telehealth visit. All patients felt that telehealth visits were more convenient than a face-to-face encounter; 53% and 41% reported that the telehealth visit saved them 1-3 hours and more than 3 hours, respectively. Ninety-four percent reported they would definitely like to have a telehealth visit in the future.
Teleconferencing
Project Extension for Community Health Care Outcomes (ECHO) was originally designed to provide specialist support for treatment of hepatitis C to primary care providers (PCPs) in rural New Mexico and the prison health system. The ECHO model leverages video teleconferencing to provide ongoing assistance from specialists to PCPs for management of cases, treatment plans, and monitoring and also provides case-based learning to increase PCP knowledge and opportunities to participate in research.25 A survey of 29 providers participating in Project ECHO revealed that more than 90% of respondents felt comfortable with management of hepatitis C as a consequence of using the program.26 Subsequently, a prospective cohort study was conducted to compare outcomes of treatment of hepatitis C between the University of New Mexico hepatitis C virus clinic and PCPs at 21 ECHO sites. In the 407Four hundred seven patients who were included in the cohort study,; sustained virologic response was obtained in 57.5% and 58.2% of patients treated at University of New Mexico and ECHO sites, respectively. Response rates did not differ by site according to genotype. Adverse events were lower at ECHO sites, compared with the hepatitis C virus clinic (6.9% vs. 13.7%, respectively).27 With a combination of funding from the state government and external funding agencies, Project ECHO now provides support from specialists to the community for 18 other chronic conditions, including but not limited to diabetes, human immunodeficiency virus, substance abuse, and high-risk pregnancies. Similar approaches have been proposed to create multidisciplinary teams for the management of hepatocellular carcinoma and cirrhosis.28
The Inflammatory Bowel Disease Live Inter-institutional and Interdisciplinary Videoconference Education (IBD LIVE) is a national weekly teleconference that brings together gastroenterologists (adult and pediatric), surgeons, radiologists, pathologists, and medical subspecialists from multiple institutions to discuss the management of complex IBD cases. IBD LIVE is continuing medical education–accredited and is published quarterly in Inflammatory Bowel Diseases. Each 1-hour conference covers two cases equally. After the initial presentation, the moderator summarizes the case and asks faculty to provide their opinion on management. Because many of the cases presented are quite complex, it is common for subspecialists from other disciplines to join the conference to provide clarity.29 The interaction among the various centers has been more than a means of obtaining continuing medical education. In many cases, referrals to other centers have been expedited because one center may offer a service not available at the “home” institution. Providers also use the summary and recommendations to help inform decisions in management of their respective patients. Exchange of ideas has resulted in collaborative research and quality improvement programs without requiring travel and/or absence from clinical duties.
Conclusions
The use of telemedicine in the care of patients with digestive diseases is expanding beyond telephone triage to include remote monitoring and self-care, telehealth visits, and teleconferencing. It is inevitable that use of these services will continue to increase to improve clinical care, to provide quality metrics, to increase access to gastroenterology care and tertiary referral expertise, to improve efficiency of health care delivery, and to decrease costs. Investment in telehealth by venture capitalists has increased fourfold from $1.1 to $4.3 billion from 2011 to 2015.30 Unfortunately, barriers to providing health care remotely continue. These include technological barriers for many practices, the requirement for providers to be licensed and credentialed in multiple states and institutions respectively, unique liability concerns, difficulties with reimbursement, and differential access to telehealth services among patients. In addition, patient engagement with remote monitoring has been disappointing, likely because of poor system designs and limited involvement with patients in design of the monitoring systems. It is likely that payers and states will slowly increase reimbursement and ease use of telemedicine as they learn how telemedicine can decrease costs and improve the efficiency of health care delivery.
Dr. Cross is in the division of gastroenterology and hepatology, department of medicine, University of Maryland, Baltimore. Dr. Kane is in the division of gastroenterology and hepatology, department of medicine, Mayo Clinic, Rochester, Minn. This research was supported by the Agency for Healthcare Research and Quality (1R01HS018975-01A1). The authors disclose no conflicts.
References
1. What is telemedicine? American Telemedicine Association. Available from http://www.americantelemed.org/main/about/about-telemedicine. Accessed September 2, 2016.
2. Ramos-Rivers, C., Regueiro, M., Vargas, E.J., et al. Association between telephone activity and features of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):986-94.
3. Patil, S.A., Cross, R.K. Can you hear me now? Frequent telephone encounters for management of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):995-6.
4. Raine L. Cell phone ownership hits 91% of adults. PewResearch Center. 2013. Available from http://www.pewresearch.org/fact-tank/2013/06/06/cell-phone-ownership-hits-91-of-adults/. Accessed September 2, 2016.
5. Ray, K.N., Chari, A.V., Engberg, J., et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175:1983-6.
6. Cross, R.K., Arora, M., Finkelstein, J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006;40(3):200-8.
7. Cross, R.K., Finkelstein, J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: A 6-month pilot study. Dig Dis Sci. 2007;52(2):357-64.
8. Cross, R.K., Cheevers, N., Rustgi, A., et al. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT). Inflamm Bowel Dis. 2012;18(6):1018-25.
9. Cross, R.K., Jambaulikar, G., Langenberg, P., et al. TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD): design and implementation of randomized clinical trial. Contemp Clin Trials. 2015;42:132-44.
10. Elkjaer, M., Burisch, J., Avnstrom, S., et al. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22(6):695-704.
11. Elkjaer, M., Shuhaibar, M., Burisch, J., et al. E-health empowers patients with ulcerative colitis: A randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652-61.
12. Pedersen, N., Elkjaer, M., Duricova, D., et al. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36(9):840-9.
13. Pedersen, N., Thielsen, P., Martinsen, L., et al. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20(12):2276-85.
14. Van Deen, W.K., van der Meulen-de Jong, A.E., Parekh, N.K., et al. Development and validation of an inflammatory bowel diseases monitoring index for use with mobile health technologies. Clin Gastroenterol Hepatol. 2016 Dec;14(12):1742-50 Nove 18.
15. van Deen, W.K., Ozbay, A.B., Skup, M., et al. The impact of a value-based health program for inflammatory bowel disease management on healthcare utilization. J Crohns Colitis. 2015;9:S123-4.
16. Atreja, A., Khan, S., Rogers, J.D., et al. Impact of the mobile HealthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease: study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc. 2015;4:e23.
17. Bajaj, J.S., Heuman, D.M., Sterling, R.K. et al. Validation of EncephalApp, smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35 e1.
18. Riaz, M.S., Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
19. Thomas L, Capistrant G. 50 state telemedicine gaps analysis physician practice standards & licensure: American Telemedicine Association. 2014. Available from http://www.americantelemed.org/docs/default-source/policy/50-state-telemedicine-gaps-analysis–physician-practice-standards-licensure.pdf. Accessed September 2, 2016.
20. Interstate Medical Licensure Compact: federation of state medical boards. Available from http://www.fsmb.org/policy/interstate-model-compact. Accessed September 2, 2016.
21. TELE-MED Act of 2015. Stat. H.R. 3081, 114th Congress (2015-2016).
22. Natoli, C.M. Summary of findings: malpractice and telemedicine. Center for Telehealth and EHealth Law, Washington, DC; 2009.
23. The National Conference of State Legislatures. Available from http://www.ncsl.org/research/health/state-coverage-for-telehealth-services.aspx. Accessed September 2, 2016.
24. Larsen E, Diamond D. Why Mayo Clinic’s CEO wants to serve 200 million patients-and how he plans to do it. 2014. Available from https://www.advisory.com/daily-briefing/2014/07/23/lessons-from-the-c-suite-mayo-clinic. Accessed September 2, 2016.
25. Arora, S., Thornton, K., Jenkusky, S.M., et al. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122:74-7.
26. Arora, S., Geppert, C.M., Kalishman, S., et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154-60.
27. Arora, S., Thornton, K., Murata, G., et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-207.
28. Naugler, W.E., Alsina, A.E., Frenette, C.T., et al. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2015;13:827-35.
29. Regueiro, M.D., Greer, J.B., Binion, D.G., et al. The inflammatory bowel disease live interinstitutional and interdisciplinary videoconference education (IBD LIVE) series. Inflamm Bowel Dis. 2014;20:1687-95.
30. Wang T., King E., Perman M., et al. Digital health funding: 2015 year in review. 2016. Available from http://rockhealth.com/reports/digital-health-funding-2015-year-in-review. Accessed. September 2, 2016.
Two trends in health care delivery that will continue unabated are a) reimbursement pressure and b) increasing demand for our services. Practices that care for patients with complex and chronic conditions are exploring innovative means to expand their care footprint in an economically viable way. One approach currently being used by many health systems is telemedicine. Telemedicine is care delivered remotely using some type of electronic communication. Potentially, telemedicine will allow us to provide specialty services remotely to primary care physicians or even patients. The University of Michigan inflammatory bowel disease program is piloting remote video conferencing, integrated within the electronic medical record system, to provide specialty gastrointestinal consultation directly to Crohn’s and ulcerative colitis patients within their homes. The University of Michigan Health System has an office ready to arrange rapid teleconsultation for any provider. Payment for services has been secured from several payers after health system negotiations. This practice is well established in multiple specialties and settings. In this month’s column, two telemedicine experts review the state of the field, so you too can participate. This innovation is something you should consider for your practice. Technology and payment mechanisms are now available.
John I. Allen, MD, MBA, AGAF
Editor in Chief
As defined by the American Telemedicine Association (ATA), telemedicine is the exchange of medical information from one site to another via electronic communication to improve a patient’s clinical health status.1 If we include care provided over the telephone via providers and nurses between office visits, telemedicine has been practiced for decades. A recent study from the University of Pittsburgh documented 32,667 phone calls from 3,118 patients with inflammatory bowel disease (IBD) in 2010. Seventy-five percent of these calls were related to patient concerns or were generated by the nurse because of changes in the treatment plan.2 If these results are applied to a representative work week, busy IBD centers typically handle more than 100 phone calls per day.3 Telemedicine in clinical practice has expanded to include a variety of modalities such as two-way video, email, or secure messaging through electronic medical records systems, smartphones, wireless tools, and other forms of telecommunication technology (see Figure 1). The increase in use of telemedicine in practice has been driven by a number of factors.
First, it is almost universal that patients have access to a computer and/or cellular telephone. According to the Pew Research Center’s Internet and American Life Project, as of May 2013, 91% of adults are using cellphones.4 As patients have become more connected digitally, it is natural that they desire delivery of services, including health care services, electronically. Second, despite advances in medical, endoscopic, and surgical treatment, many patients still have suboptimal outcomes. There are many reasons for this, including but not limited to nonadherence, poor patient education, inadequate monitoring of symptoms and side effects, concurrent psychiatric disease, comorbid medical conditions, low self-efficacy, and limited access to health care; these issues can be addressed, at least in part, by telemedicine. Finally, patients are also seeking more efficient and convenient ways to receive their care,; including travel and wait times, an average office visit takes up to 2 hours.5
Enhanced monitoring and self-care through use of telemedicine technologies
Several groups have implemented telemedicine to improve monitoring and self-care in patients with IBD. Our group at the University of Maryland, Baltimore, has developed several systems to improve care as part of research protocols. Our first telemedicine system included a laptop computer and electronic weight scale connected telephonically to a server. Patients were asked questions about bowel symptoms, medication use, side effects, and body weight measurements. They also received educational messages. This system, IBD Home Automated Telemanagement (HAT), required installation in the patient’s home by a technical team. Our preliminary results demonstrated that patients were very receptive of the technology.6 In a small pilot study (n = 34), we demonstrated that 88% of patients were adherent to self-assessment during a period of 6 months. In addition, patients experienced a reduction in disease activity, improved quality of life, and increased disease state awareness.7 In a small, randomized, controlled follow-up trial, we demonstrated that use of an ulcerative colitis (UC) telemanagement system (UC HAT) resulted in improved quality of life and decreased disease activity from baseline during 1 year compared with controls. The UC HAT system was enhanced to include self-care plans that were based on patient reporting of symptoms. Fewer participants completed the study in the UC HAT, compared with the control, group (56% vs 72%).8 We theorized that participant dropout was higher in the UC HAT group because of the requirement for a technician to visit the home to install or service the system. Hence, as part of a randomized controlled trial, our group has collaborated with the University of Pittsburgh and Vanderbilt University to assess a new telemedicine system that monitors patients by using text messaging.9 Three hundred forty-eight patients were recruited for this ongoing clinical trial. Thus far, 83%–84% of participants in the intervention arms have completed the 1-year study.
Elkjaer et al. evaluated the impact of a web-based treatment program and patient education center in a convenience sample of patients with UC.10 All 21 patients reported the ability to initiate a self-care plan. Furthermore, participants experienced improvements in knowledge after interaction with the patient education center.10 The web-based self-management and treatment approach was compared with standard of care in 333 patients with mild-to-moderate UC from Ireland and Denmark.11 Only 135 patients (41%) completed the 1-year study. Web subjects were more adherent with acute treatment, demonstrated improved disease knowledge and quality of life, experienced shorter relapses, and had fewer office and urgent care visits. Conversely, web group patients generated more emails and telephone calls. In the Irish arm, the results were similar; however, there was no difference in quality of life between groups, and the relapse rate was higher in the web group, compared with controls.11 A 2012 study by the same group investigated the efficacy of web-based monitoring of Crohn’s disease activity for individualized dosing of infliximab maintenance therapy. Twenty-seven patients were enrolled; 17 completed 52 weeks and 6 completed 26 weeks of follow-up. Patients recorded their symptoms weekly via a web-based portal; on the basis of symptom scores, patients were instructed to contact their physician for an infliximab infusion. Fifty percent of the patients were able to tolerate intervals greater than 8 weeks, whereas 36% required shorter intervals.12 This concept of web-based personalized treatment was further investigated in a 2014 study evaluating 86 patients with mild-to-moderate UC. Mesalamine treatment was individualized on the basis of a composite index of clinical symptoms and fecal calprotectin levels. Use of the web application was associated with decreased disease activity scores and lower fecal calprotectin levels despite dose reduction in 88% of patients at week 12.13
The eIBD program developed at the University of California, Los Angeles, also uses a web-based platform to monitor patients. After an initial training session with an IBD nurse specialist, patients are able to view clinical results and view and update their disease activity status, quality of life, and work productivity remotely. Patients interact with the eIBD program by using a tablet or home computer. Self-monitoring was found to correlate well with an in-person assessment of symptoms and disease activity.14 Patient care is organized into evidence-based pathways on the basis of disease status and the medication regimen. Each pathway has a defined number of clinic and electronic visits and laboratory sets. Patients can also access support programs such as My Academy, My Work, My Coach, My Physical Fitness, and My Diet. When University of California, Los Angeles, IBD patients were compared with matched controls by using an administrative claims database, they were significantly less likely to use steroids and had fewer hospitalizations and emergency department visits.15
HealthPROMISE is an application developed at Mount Sinai to collect patient-reported outcomes and to provide decision support. Patient-reported symptoms and quality of life are integrated into the electronic medical records system; providers can view the information in real time to better manage their panel of patients. HealthPROMISE is currently being evaluated as part of a pragmatic, multicenter, randomized controlled trial.16 EncephalApp is a mobile phone application used to assess patients for hepatic encephalopathy. The application was tested in 167 cirrhotic patients, 38% of whom had overt encephalopathy, and 114 controls. The application was shown to have excellent discriminant ability to detect encephalopathy, and importantly, EnecphalApp times correlated with motor vehicle accidents and illegal turns in a driving simulation test.17 A number of other mobile applications have been developed to support patients with chronic illnesses. These applications can be integrated with wearable devices, and some have been approved by the Food and Drug Administration.18
Telehealth and teleconsultation
Advancements in telemedicine have outpaced the ability of legislators and institutional officials to provide oversight on legal and regulatory issues. Each state sets requirements for providers to engage in telehealth activities. The ATA published the State Telemedicine Gap Analysis to address specific requirements and limitations for each state.19 Issues addressed in this document include the requirement for a face-to-face visit before a telehealth visit, informed consent, and interstate practice. Virtually all states have barriers to providing telemedicine services unless the provider is licensed in the state where the patient resides. To promote telemedicine, the Federation of State Medical Boards proposed the development of an Interstate Licensure Compact in which 17 states participate (AL, AZ, CO, ID, IL, IA, KS, MN, MS, MT, NV, NH, SD, UT, WV, WI, and WY).20 Two key principles include defining the practice of medicine as the location in which the patient resides and placing the provider under the jurisdiction of the state in which the practice occurs. The TELE-MED Act of 2015 has proposed allowing Medicare physicians to provide telehealth services to patients regardless of the state in which they reside.21 In regard to liability, the number of malpractice cases involving telemedicine services is low; most are related to e-prescribing, as opposed to care provided during teleconsultation services.22
However, some unique liability issues relative to telehealth encounters exist. First, when considering standard of care, what do you compare a telemedicine encounter with? Hardware or software malfunctions can occur, with a subsequent inability to provide the telemedicine service. Loss of protected health information through hackers or equipment failure is another potential threat. Reimbursement for telehealth services is also regulated by states and is subject to wide variability; 29 states have laws in place requiring private payers to reimburse for telehealth services at the same level as an in-person encounter.19 The ATA recently published an analysis of issues related to reimbursements.23 In addition to parity, key issues that must be addressed include the failure of the majority of state health plans to provide coverage for telehealth services to employees, restrictions on providing telehealth in nonrural settings, restrictions that are based on the type of health care provider, and restrictions on home monitoring.
The Mayo Clinic, Rochester, Minn., offers outreach to its health system affiliates via a secure video conferencing platform to allow face-to-face consultations for patients with IBD. Consultative appointments are scheduled during preassigned blocks of outreach time on the clinician’s calendar. A nurse transcribes any recommendation that requires an order from the referring gastroenterologist. Health care providers offering video consultation are required to have a medical license for the state in which the patient resides and to be credentialed by the facility to which the Mayo Clinic provides services and the payer reimbursing for the service. The majority of consultations are for discussions regarding medical management: when to start a biologic, safety concerns, monitoring strategies, or for a second opinion regarding the need for surgery for refractory UC or fibrostenotic Crohn’s disease. Access to imaging and laboratory work is facilitated through previsit evaluation performed by a nurse in the referring practice.
If services are provided via consultation to a patient at a non–Mayo Clinic facility via the Affiliated Care Network, there is a legal contract outlining reimbursement as well as terms and conditions. For asynchronous consultation where there is interaction with a provider but the patient is not directly involved (no face-to-face consultation), the practice of medicine regulations vary from state to state as outlined above. Credentialing is not required for provider-to-provider consultation at each site but sometimes a license is. However, for all states, electronic health record documentation of the clinical question and recommendations is important. Substantial administrative infrastructure is required to manage the quality of e-consult responses so that they are timely and the clinical notes meet the needs of the requesting provider. This is supported by a secure online portal that exchanges electronic health record information and the clinical note generated. Mayo Clinic providers are licensed for multiple states to provide medical consultations to various affiliated hospitals throughout the United States. The consulting physician always has the option to recommend a full face-to-face consultation if review of the records provided indicates the patient appears to be too complicated. The telehealth efforts at the Mayo Clinic are not isolated to gastroenterology; it is estimated that, through expanded use of telehealth, Mayo Clinic will provide care nationally and internationally for 200 million people by 2020.24
From April 2015 to May 2016, at the University of Maryland, Baltimore, we conducted 89 telehealth visits. According to state regulations and payer restrictions on reimbursement, patients were eligible to undergo telehealth visits if they had a prior face-to-face visit and were insured by Blue Cross Blue Shield. Eligible patients provided informed consent to participate in the telehealth visit. Patients received an email with instructions on how to download the required software (VidyoDesktop version 3.0.4[001]; Vidyo, Hackensack, N.J.) onto the patient’s home computer, tablet, or smartphone. An office assistant conducted a test visit to make sure that the connection was adequate. Eighty-three percent of patients reported that using the system was not complicated at all or only slightly complicated. Seventy-one percent reported that the telehealth visit took significantly less or less time than a routine encounter; 88% said that all their concerns were addressed during the telehealth visit. All patients felt that telehealth visits were more convenient than a face-to-face encounter; 53% and 41% reported that the telehealth visit saved them 1-3 hours and more than 3 hours, respectively. Ninety-four percent reported they would definitely like to have a telehealth visit in the future.
Teleconferencing
Project Extension for Community Health Care Outcomes (ECHO) was originally designed to provide specialist support for treatment of hepatitis C to primary care providers (PCPs) in rural New Mexico and the prison health system. The ECHO model leverages video teleconferencing to provide ongoing assistance from specialists to PCPs for management of cases, treatment plans, and monitoring and also provides case-based learning to increase PCP knowledge and opportunities to participate in research.25 A survey of 29 providers participating in Project ECHO revealed that more than 90% of respondents felt comfortable with management of hepatitis C as a consequence of using the program.26 Subsequently, a prospective cohort study was conducted to compare outcomes of treatment of hepatitis C between the University of New Mexico hepatitis C virus clinic and PCPs at 21 ECHO sites. In the 407Four hundred seven patients who were included in the cohort study,; sustained virologic response was obtained in 57.5% and 58.2% of patients treated at University of New Mexico and ECHO sites, respectively. Response rates did not differ by site according to genotype. Adverse events were lower at ECHO sites, compared with the hepatitis C virus clinic (6.9% vs. 13.7%, respectively).27 With a combination of funding from the state government and external funding agencies, Project ECHO now provides support from specialists to the community for 18 other chronic conditions, including but not limited to diabetes, human immunodeficiency virus, substance abuse, and high-risk pregnancies. Similar approaches have been proposed to create multidisciplinary teams for the management of hepatocellular carcinoma and cirrhosis.28
The Inflammatory Bowel Disease Live Inter-institutional and Interdisciplinary Videoconference Education (IBD LIVE) is a national weekly teleconference that brings together gastroenterologists (adult and pediatric), surgeons, radiologists, pathologists, and medical subspecialists from multiple institutions to discuss the management of complex IBD cases. IBD LIVE is continuing medical education–accredited and is published quarterly in Inflammatory Bowel Diseases. Each 1-hour conference covers two cases equally. After the initial presentation, the moderator summarizes the case and asks faculty to provide their opinion on management. Because many of the cases presented are quite complex, it is common for subspecialists from other disciplines to join the conference to provide clarity.29 The interaction among the various centers has been more than a means of obtaining continuing medical education. In many cases, referrals to other centers have been expedited because one center may offer a service not available at the “home” institution. Providers also use the summary and recommendations to help inform decisions in management of their respective patients. Exchange of ideas has resulted in collaborative research and quality improvement programs without requiring travel and/or absence from clinical duties.
Conclusions
The use of telemedicine in the care of patients with digestive diseases is expanding beyond telephone triage to include remote monitoring and self-care, telehealth visits, and teleconferencing. It is inevitable that use of these services will continue to increase to improve clinical care, to provide quality metrics, to increase access to gastroenterology care and tertiary referral expertise, to improve efficiency of health care delivery, and to decrease costs. Investment in telehealth by venture capitalists has increased fourfold from $1.1 to $4.3 billion from 2011 to 2015.30 Unfortunately, barriers to providing health care remotely continue. These include technological barriers for many practices, the requirement for providers to be licensed and credentialed in multiple states and institutions respectively, unique liability concerns, difficulties with reimbursement, and differential access to telehealth services among patients. In addition, patient engagement with remote monitoring has been disappointing, likely because of poor system designs and limited involvement with patients in design of the monitoring systems. It is likely that payers and states will slowly increase reimbursement and ease use of telemedicine as they learn how telemedicine can decrease costs and improve the efficiency of health care delivery.
Dr. Cross is in the division of gastroenterology and hepatology, department of medicine, University of Maryland, Baltimore. Dr. Kane is in the division of gastroenterology and hepatology, department of medicine, Mayo Clinic, Rochester, Minn. This research was supported by the Agency for Healthcare Research and Quality (1R01HS018975-01A1). The authors disclose no conflicts.
References
1. What is telemedicine? American Telemedicine Association. Available from http://www.americantelemed.org/main/about/about-telemedicine. Accessed September 2, 2016.
2. Ramos-Rivers, C., Regueiro, M., Vargas, E.J., et al. Association between telephone activity and features of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):986-94.
3. Patil, S.A., Cross, R.K. Can you hear me now? Frequent telephone encounters for management of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):995-6.
4. Raine L. Cell phone ownership hits 91% of adults. PewResearch Center. 2013. Available from http://www.pewresearch.org/fact-tank/2013/06/06/cell-phone-ownership-hits-91-of-adults/. Accessed September 2, 2016.
5. Ray, K.N., Chari, A.V., Engberg, J., et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175:1983-6.
6. Cross, R.K., Arora, M., Finkelstein, J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006;40(3):200-8.
7. Cross, R.K., Finkelstein, J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: A 6-month pilot study. Dig Dis Sci. 2007;52(2):357-64.
8. Cross, R.K., Cheevers, N., Rustgi, A., et al. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT). Inflamm Bowel Dis. 2012;18(6):1018-25.
9. Cross, R.K., Jambaulikar, G., Langenberg, P., et al. TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD): design and implementation of randomized clinical trial. Contemp Clin Trials. 2015;42:132-44.
10. Elkjaer, M., Burisch, J., Avnstrom, S., et al. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22(6):695-704.
11. Elkjaer, M., Shuhaibar, M., Burisch, J., et al. E-health empowers patients with ulcerative colitis: A randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652-61.
12. Pedersen, N., Elkjaer, M., Duricova, D., et al. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36(9):840-9.
13. Pedersen, N., Thielsen, P., Martinsen, L., et al. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20(12):2276-85.
14. Van Deen, W.K., van der Meulen-de Jong, A.E., Parekh, N.K., et al. Development and validation of an inflammatory bowel diseases monitoring index for use with mobile health technologies. Clin Gastroenterol Hepatol. 2016 Dec;14(12):1742-50 Nove 18.
15. van Deen, W.K., Ozbay, A.B., Skup, M., et al. The impact of a value-based health program for inflammatory bowel disease management on healthcare utilization. J Crohns Colitis. 2015;9:S123-4.
16. Atreja, A., Khan, S., Rogers, J.D., et al. Impact of the mobile HealthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease: study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc. 2015;4:e23.
17. Bajaj, J.S., Heuman, D.M., Sterling, R.K. et al. Validation of EncephalApp, smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35 e1.
18. Riaz, M.S., Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
19. Thomas L, Capistrant G. 50 state telemedicine gaps analysis physician practice standards & licensure: American Telemedicine Association. 2014. Available from http://www.americantelemed.org/docs/default-source/policy/50-state-telemedicine-gaps-analysis–physician-practice-standards-licensure.pdf. Accessed September 2, 2016.
20. Interstate Medical Licensure Compact: federation of state medical boards. Available from http://www.fsmb.org/policy/interstate-model-compact. Accessed September 2, 2016.
21. TELE-MED Act of 2015. Stat. H.R. 3081, 114th Congress (2015-2016).
22. Natoli, C.M. Summary of findings: malpractice and telemedicine. Center for Telehealth and EHealth Law, Washington, DC; 2009.
23. The National Conference of State Legislatures. Available from http://www.ncsl.org/research/health/state-coverage-for-telehealth-services.aspx. Accessed September 2, 2016.
24. Larsen E, Diamond D. Why Mayo Clinic’s CEO wants to serve 200 million patients-and how he plans to do it. 2014. Available from https://www.advisory.com/daily-briefing/2014/07/23/lessons-from-the-c-suite-mayo-clinic. Accessed September 2, 2016.
25. Arora, S., Thornton, K., Jenkusky, S.M., et al. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122:74-7.
26. Arora, S., Geppert, C.M., Kalishman, S., et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154-60.
27. Arora, S., Thornton, K., Murata, G., et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-207.
28. Naugler, W.E., Alsina, A.E., Frenette, C.T., et al. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2015;13:827-35.
29. Regueiro, M.D., Greer, J.B., Binion, D.G., et al. The inflammatory bowel disease live interinstitutional and interdisciplinary videoconference education (IBD LIVE) series. Inflamm Bowel Dis. 2014;20:1687-95.
30. Wang T., King E., Perman M., et al. Digital health funding: 2015 year in review. 2016. Available from http://rockhealth.com/reports/digital-health-funding-2015-year-in-review. Accessed. September 2, 2016.
Two trends in health care delivery that will continue unabated are a) reimbursement pressure and b) increasing demand for our services. Practices that care for patients with complex and chronic conditions are exploring innovative means to expand their care footprint in an economically viable way. One approach currently being used by many health systems is telemedicine. Telemedicine is care delivered remotely using some type of electronic communication. Potentially, telemedicine will allow us to provide specialty services remotely to primary care physicians or even patients. The University of Michigan inflammatory bowel disease program is piloting remote video conferencing, integrated within the electronic medical record system, to provide specialty gastrointestinal consultation directly to Crohn’s and ulcerative colitis patients within their homes. The University of Michigan Health System has an office ready to arrange rapid teleconsultation for any provider. Payment for services has been secured from several payers after health system negotiations. This practice is well established in multiple specialties and settings. In this month’s column, two telemedicine experts review the state of the field, so you too can participate. This innovation is something you should consider for your practice. Technology and payment mechanisms are now available.
John I. Allen, MD, MBA, AGAF
Editor in Chief
As defined by the American Telemedicine Association (ATA), telemedicine is the exchange of medical information from one site to another via electronic communication to improve a patient’s clinical health status.1 If we include care provided over the telephone via providers and nurses between office visits, telemedicine has been practiced for decades. A recent study from the University of Pittsburgh documented 32,667 phone calls from 3,118 patients with inflammatory bowel disease (IBD) in 2010. Seventy-five percent of these calls were related to patient concerns or were generated by the nurse because of changes in the treatment plan.2 If these results are applied to a representative work week, busy IBD centers typically handle more than 100 phone calls per day.3 Telemedicine in clinical practice has expanded to include a variety of modalities such as two-way video, email, or secure messaging through electronic medical records systems, smartphones, wireless tools, and other forms of telecommunication technology (see Figure 1). The increase in use of telemedicine in practice has been driven by a number of factors.
First, it is almost universal that patients have access to a computer and/or cellular telephone. According to the Pew Research Center’s Internet and American Life Project, as of May 2013, 91% of adults are using cellphones.4 As patients have become more connected digitally, it is natural that they desire delivery of services, including health care services, electronically. Second, despite advances in medical, endoscopic, and surgical treatment, many patients still have suboptimal outcomes. There are many reasons for this, including but not limited to nonadherence, poor patient education, inadequate monitoring of symptoms and side effects, concurrent psychiatric disease, comorbid medical conditions, low self-efficacy, and limited access to health care; these issues can be addressed, at least in part, by telemedicine. Finally, patients are also seeking more efficient and convenient ways to receive their care,; including travel and wait times, an average office visit takes up to 2 hours.5
Enhanced monitoring and self-care through use of telemedicine technologies
Several groups have implemented telemedicine to improve monitoring and self-care in patients with IBD. Our group at the University of Maryland, Baltimore, has developed several systems to improve care as part of research protocols. Our first telemedicine system included a laptop computer and electronic weight scale connected telephonically to a server. Patients were asked questions about bowel symptoms, medication use, side effects, and body weight measurements. They also received educational messages. This system, IBD Home Automated Telemanagement (HAT), required installation in the patient’s home by a technical team. Our preliminary results demonstrated that patients were very receptive of the technology.6 In a small pilot study (n = 34), we demonstrated that 88% of patients were adherent to self-assessment during a period of 6 months. In addition, patients experienced a reduction in disease activity, improved quality of life, and increased disease state awareness.7 In a small, randomized, controlled follow-up trial, we demonstrated that use of an ulcerative colitis (UC) telemanagement system (UC HAT) resulted in improved quality of life and decreased disease activity from baseline during 1 year compared with controls. The UC HAT system was enhanced to include self-care plans that were based on patient reporting of symptoms. Fewer participants completed the study in the UC HAT, compared with the control, group (56% vs 72%).8 We theorized that participant dropout was higher in the UC HAT group because of the requirement for a technician to visit the home to install or service the system. Hence, as part of a randomized controlled trial, our group has collaborated with the University of Pittsburgh and Vanderbilt University to assess a new telemedicine system that monitors patients by using text messaging.9 Three hundred forty-eight patients were recruited for this ongoing clinical trial. Thus far, 83%–84% of participants in the intervention arms have completed the 1-year study.
Elkjaer et al. evaluated the impact of a web-based treatment program and patient education center in a convenience sample of patients with UC.10 All 21 patients reported the ability to initiate a self-care plan. Furthermore, participants experienced improvements in knowledge after interaction with the patient education center.10 The web-based self-management and treatment approach was compared with standard of care in 333 patients with mild-to-moderate UC from Ireland and Denmark.11 Only 135 patients (41%) completed the 1-year study. Web subjects were more adherent with acute treatment, demonstrated improved disease knowledge and quality of life, experienced shorter relapses, and had fewer office and urgent care visits. Conversely, web group patients generated more emails and telephone calls. In the Irish arm, the results were similar; however, there was no difference in quality of life between groups, and the relapse rate was higher in the web group, compared with controls.11 A 2012 study by the same group investigated the efficacy of web-based monitoring of Crohn’s disease activity for individualized dosing of infliximab maintenance therapy. Twenty-seven patients were enrolled; 17 completed 52 weeks and 6 completed 26 weeks of follow-up. Patients recorded their symptoms weekly via a web-based portal; on the basis of symptom scores, patients were instructed to contact their physician for an infliximab infusion. Fifty percent of the patients were able to tolerate intervals greater than 8 weeks, whereas 36% required shorter intervals.12 This concept of web-based personalized treatment was further investigated in a 2014 study evaluating 86 patients with mild-to-moderate UC. Mesalamine treatment was individualized on the basis of a composite index of clinical symptoms and fecal calprotectin levels. Use of the web application was associated with decreased disease activity scores and lower fecal calprotectin levels despite dose reduction in 88% of patients at week 12.13
The eIBD program developed at the University of California, Los Angeles, also uses a web-based platform to monitor patients. After an initial training session with an IBD nurse specialist, patients are able to view clinical results and view and update their disease activity status, quality of life, and work productivity remotely. Patients interact with the eIBD program by using a tablet or home computer. Self-monitoring was found to correlate well with an in-person assessment of symptoms and disease activity.14 Patient care is organized into evidence-based pathways on the basis of disease status and the medication regimen. Each pathway has a defined number of clinic and electronic visits and laboratory sets. Patients can also access support programs such as My Academy, My Work, My Coach, My Physical Fitness, and My Diet. When University of California, Los Angeles, IBD patients were compared with matched controls by using an administrative claims database, they were significantly less likely to use steroids and had fewer hospitalizations and emergency department visits.15
HealthPROMISE is an application developed at Mount Sinai to collect patient-reported outcomes and to provide decision support. Patient-reported symptoms and quality of life are integrated into the electronic medical records system; providers can view the information in real time to better manage their panel of patients. HealthPROMISE is currently being evaluated as part of a pragmatic, multicenter, randomized controlled trial.16 EncephalApp is a mobile phone application used to assess patients for hepatic encephalopathy. The application was tested in 167 cirrhotic patients, 38% of whom had overt encephalopathy, and 114 controls. The application was shown to have excellent discriminant ability to detect encephalopathy, and importantly, EnecphalApp times correlated with motor vehicle accidents and illegal turns in a driving simulation test.17 A number of other mobile applications have been developed to support patients with chronic illnesses. These applications can be integrated with wearable devices, and some have been approved by the Food and Drug Administration.18
Telehealth and teleconsultation
Advancements in telemedicine have outpaced the ability of legislators and institutional officials to provide oversight on legal and regulatory issues. Each state sets requirements for providers to engage in telehealth activities. The ATA published the State Telemedicine Gap Analysis to address specific requirements and limitations for each state.19 Issues addressed in this document include the requirement for a face-to-face visit before a telehealth visit, informed consent, and interstate practice. Virtually all states have barriers to providing telemedicine services unless the provider is licensed in the state where the patient resides. To promote telemedicine, the Federation of State Medical Boards proposed the development of an Interstate Licensure Compact in which 17 states participate (AL, AZ, CO, ID, IL, IA, KS, MN, MS, MT, NV, NH, SD, UT, WV, WI, and WY).20 Two key principles include defining the practice of medicine as the location in which the patient resides and placing the provider under the jurisdiction of the state in which the practice occurs. The TELE-MED Act of 2015 has proposed allowing Medicare physicians to provide telehealth services to patients regardless of the state in which they reside.21 In regard to liability, the number of malpractice cases involving telemedicine services is low; most are related to e-prescribing, as opposed to care provided during teleconsultation services.22
However, some unique liability issues relative to telehealth encounters exist. First, when considering standard of care, what do you compare a telemedicine encounter with? Hardware or software malfunctions can occur, with a subsequent inability to provide the telemedicine service. Loss of protected health information through hackers or equipment failure is another potential threat. Reimbursement for telehealth services is also regulated by states and is subject to wide variability; 29 states have laws in place requiring private payers to reimburse for telehealth services at the same level as an in-person encounter.19 The ATA recently published an analysis of issues related to reimbursements.23 In addition to parity, key issues that must be addressed include the failure of the majority of state health plans to provide coverage for telehealth services to employees, restrictions on providing telehealth in nonrural settings, restrictions that are based on the type of health care provider, and restrictions on home monitoring.
The Mayo Clinic, Rochester, Minn., offers outreach to its health system affiliates via a secure video conferencing platform to allow face-to-face consultations for patients with IBD. Consultative appointments are scheduled during preassigned blocks of outreach time on the clinician’s calendar. A nurse transcribes any recommendation that requires an order from the referring gastroenterologist. Health care providers offering video consultation are required to have a medical license for the state in which the patient resides and to be credentialed by the facility to which the Mayo Clinic provides services and the payer reimbursing for the service. The majority of consultations are for discussions regarding medical management: when to start a biologic, safety concerns, monitoring strategies, or for a second opinion regarding the need for surgery for refractory UC or fibrostenotic Crohn’s disease. Access to imaging and laboratory work is facilitated through previsit evaluation performed by a nurse in the referring practice.
If services are provided via consultation to a patient at a non–Mayo Clinic facility via the Affiliated Care Network, there is a legal contract outlining reimbursement as well as terms and conditions. For asynchronous consultation where there is interaction with a provider but the patient is not directly involved (no face-to-face consultation), the practice of medicine regulations vary from state to state as outlined above. Credentialing is not required for provider-to-provider consultation at each site but sometimes a license is. However, for all states, electronic health record documentation of the clinical question and recommendations is important. Substantial administrative infrastructure is required to manage the quality of e-consult responses so that they are timely and the clinical notes meet the needs of the requesting provider. This is supported by a secure online portal that exchanges electronic health record information and the clinical note generated. Mayo Clinic providers are licensed for multiple states to provide medical consultations to various affiliated hospitals throughout the United States. The consulting physician always has the option to recommend a full face-to-face consultation if review of the records provided indicates the patient appears to be too complicated. The telehealth efforts at the Mayo Clinic are not isolated to gastroenterology; it is estimated that, through expanded use of telehealth, Mayo Clinic will provide care nationally and internationally for 200 million people by 2020.24
From April 2015 to May 2016, at the University of Maryland, Baltimore, we conducted 89 telehealth visits. According to state regulations and payer restrictions on reimbursement, patients were eligible to undergo telehealth visits if they had a prior face-to-face visit and were insured by Blue Cross Blue Shield. Eligible patients provided informed consent to participate in the telehealth visit. Patients received an email with instructions on how to download the required software (VidyoDesktop version 3.0.4[001]; Vidyo, Hackensack, N.J.) onto the patient’s home computer, tablet, or smartphone. An office assistant conducted a test visit to make sure that the connection was adequate. Eighty-three percent of patients reported that using the system was not complicated at all or only slightly complicated. Seventy-one percent reported that the telehealth visit took significantly less or less time than a routine encounter; 88% said that all their concerns were addressed during the telehealth visit. All patients felt that telehealth visits were more convenient than a face-to-face encounter; 53% and 41% reported that the telehealth visit saved them 1-3 hours and more than 3 hours, respectively. Ninety-four percent reported they would definitely like to have a telehealth visit in the future.
Teleconferencing
Project Extension for Community Health Care Outcomes (ECHO) was originally designed to provide specialist support for treatment of hepatitis C to primary care providers (PCPs) in rural New Mexico and the prison health system. The ECHO model leverages video teleconferencing to provide ongoing assistance from specialists to PCPs for management of cases, treatment plans, and monitoring and also provides case-based learning to increase PCP knowledge and opportunities to participate in research.25 A survey of 29 providers participating in Project ECHO revealed that more than 90% of respondents felt comfortable with management of hepatitis C as a consequence of using the program.26 Subsequently, a prospective cohort study was conducted to compare outcomes of treatment of hepatitis C between the University of New Mexico hepatitis C virus clinic and PCPs at 21 ECHO sites. In the 407Four hundred seven patients who were included in the cohort study,; sustained virologic response was obtained in 57.5% and 58.2% of patients treated at University of New Mexico and ECHO sites, respectively. Response rates did not differ by site according to genotype. Adverse events were lower at ECHO sites, compared with the hepatitis C virus clinic (6.9% vs. 13.7%, respectively).27 With a combination of funding from the state government and external funding agencies, Project ECHO now provides support from specialists to the community for 18 other chronic conditions, including but not limited to diabetes, human immunodeficiency virus, substance abuse, and high-risk pregnancies. Similar approaches have been proposed to create multidisciplinary teams for the management of hepatocellular carcinoma and cirrhosis.28
The Inflammatory Bowel Disease Live Inter-institutional and Interdisciplinary Videoconference Education (IBD LIVE) is a national weekly teleconference that brings together gastroenterologists (adult and pediatric), surgeons, radiologists, pathologists, and medical subspecialists from multiple institutions to discuss the management of complex IBD cases. IBD LIVE is continuing medical education–accredited and is published quarterly in Inflammatory Bowel Diseases. Each 1-hour conference covers two cases equally. After the initial presentation, the moderator summarizes the case and asks faculty to provide their opinion on management. Because many of the cases presented are quite complex, it is common for subspecialists from other disciplines to join the conference to provide clarity.29 The interaction among the various centers has been more than a means of obtaining continuing medical education. In many cases, referrals to other centers have been expedited because one center may offer a service not available at the “home” institution. Providers also use the summary and recommendations to help inform decisions in management of their respective patients. Exchange of ideas has resulted in collaborative research and quality improvement programs without requiring travel and/or absence from clinical duties.
Conclusions
The use of telemedicine in the care of patients with digestive diseases is expanding beyond telephone triage to include remote monitoring and self-care, telehealth visits, and teleconferencing. It is inevitable that use of these services will continue to increase to improve clinical care, to provide quality metrics, to increase access to gastroenterology care and tertiary referral expertise, to improve efficiency of health care delivery, and to decrease costs. Investment in telehealth by venture capitalists has increased fourfold from $1.1 to $4.3 billion from 2011 to 2015.30 Unfortunately, barriers to providing health care remotely continue. These include technological barriers for many practices, the requirement for providers to be licensed and credentialed in multiple states and institutions respectively, unique liability concerns, difficulties with reimbursement, and differential access to telehealth services among patients. In addition, patient engagement with remote monitoring has been disappointing, likely because of poor system designs and limited involvement with patients in design of the monitoring systems. It is likely that payers and states will slowly increase reimbursement and ease use of telemedicine as they learn how telemedicine can decrease costs and improve the efficiency of health care delivery.
Dr. Cross is in the division of gastroenterology and hepatology, department of medicine, University of Maryland, Baltimore. Dr. Kane is in the division of gastroenterology and hepatology, department of medicine, Mayo Clinic, Rochester, Minn. This research was supported by the Agency for Healthcare Research and Quality (1R01HS018975-01A1). The authors disclose no conflicts.
References
1. What is telemedicine? American Telemedicine Association. Available from http://www.americantelemed.org/main/about/about-telemedicine. Accessed September 2, 2016.
2. Ramos-Rivers, C., Regueiro, M., Vargas, E.J., et al. Association between telephone activity and features of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):986-94.
3. Patil, S.A., Cross, R.K. Can you hear me now? Frequent telephone encounters for management of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(6):995-6.
4. Raine L. Cell phone ownership hits 91% of adults. PewResearch Center. 2013. Available from http://www.pewresearch.org/fact-tank/2013/06/06/cell-phone-ownership-hits-91-of-adults/. Accessed September 2, 2016.
5. Ray, K.N., Chari, A.V., Engberg, J., et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175:1983-6.
6. Cross, R.K., Arora, M., Finkelstein, J. Acceptance of telemanagement is high in patients with inflammatory bowel disease. J Clin Gastroenterol. 2006;40(3):200-8.
7. Cross, R.K., Finkelstein, J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: A 6-month pilot study. Dig Dis Sci. 2007;52(2):357-64.
8. Cross, R.K., Cheevers, N., Rustgi, A., et al. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT). Inflamm Bowel Dis. 2012;18(6):1018-25.
9. Cross, R.K., Jambaulikar, G., Langenberg, P., et al. TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD): design and implementation of randomized clinical trial. Contemp Clin Trials. 2015;42:132-44.
10. Elkjaer, M., Burisch, J., Avnstrom, S., et al. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22(6):695-704.
11. Elkjaer, M., Shuhaibar, M., Burisch, J., et al. E-health empowers patients with ulcerative colitis: A randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652-61.
12. Pedersen, N., Elkjaer, M., Duricova, D., et al. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36(9):840-9.
13. Pedersen, N., Thielsen, P., Martinsen, L., et al. eHealth: individualization of mesalazine treatment through a self-managed web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014;20(12):2276-85.
14. Van Deen, W.K., van der Meulen-de Jong, A.E., Parekh, N.K., et al. Development and validation of an inflammatory bowel diseases monitoring index for use with mobile health technologies. Clin Gastroenterol Hepatol. 2016 Dec;14(12):1742-50 Nove 18.
15. van Deen, W.K., Ozbay, A.B., Skup, M., et al. The impact of a value-based health program for inflammatory bowel disease management on healthcare utilization. J Crohns Colitis. 2015;9:S123-4.
16. Atreja, A., Khan, S., Rogers, J.D., et al. Impact of the mobile HealthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease: study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc. 2015;4:e23.
17. Bajaj, J.S., Heuman, D.M., Sterling, R.K. et al. Validation of EncephalApp, smartphone-based Stroop test, for the diagnosis of covert hepatic encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-35 e1.
18. Riaz, M.S., Atreja, A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol. 2016;14:1697-705.
19. Thomas L, Capistrant G. 50 state telemedicine gaps analysis physician practice standards & licensure: American Telemedicine Association. 2014. Available from http://www.americantelemed.org/docs/default-source/policy/50-state-telemedicine-gaps-analysis–physician-practice-standards-licensure.pdf. Accessed September 2, 2016.
20. Interstate Medical Licensure Compact: federation of state medical boards. Available from http://www.fsmb.org/policy/interstate-model-compact. Accessed September 2, 2016.
21. TELE-MED Act of 2015. Stat. H.R. 3081, 114th Congress (2015-2016).
22. Natoli, C.M. Summary of findings: malpractice and telemedicine. Center for Telehealth and EHealth Law, Washington, DC; 2009.
23. The National Conference of State Legislatures. Available from http://www.ncsl.org/research/health/state-coverage-for-telehealth-services.aspx. Accessed September 2, 2016.
24. Larsen E, Diamond D. Why Mayo Clinic’s CEO wants to serve 200 million patients-and how he plans to do it. 2014. Available from https://www.advisory.com/daily-briefing/2014/07/23/lessons-from-the-c-suite-mayo-clinic. Accessed September 2, 2016.
25. Arora, S., Thornton, K., Jenkusky, S.M., et al. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122:74-7.
26. Arora, S., Geppert, C.M., Kalishman, S., et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154-60.
27. Arora, S., Thornton, K., Murata, G., et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-207.
28. Naugler, W.E., Alsina, A.E., Frenette, C.T., et al. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2015;13:827-35.
29. Regueiro, M.D., Greer, J.B., Binion, D.G., et al. The inflammatory bowel disease live interinstitutional and interdisciplinary videoconference education (IBD LIVE) series. Inflamm Bowel Dis. 2014;20:1687-95.
30. Wang T., King E., Perman M., et al. Digital health funding: 2015 year in review. 2016. Available from http://rockhealth.com/reports/digital-health-funding-2015-year-in-review. Accessed. September 2, 2016.
Approach to covert recording by patients of encounters with gastroenterology providers
Last year, while sedated for colonoscopy, a patient covertly recorded conversations between endoscopy staff and providers. Comments about the patient were egregious and resulted in loss of employment for those involved and a large financial settlement. The reality of today’s world is that we all are subject to constant (real or potential) surveillance. Nothing is private and nothing recorded is temporary, yet physicians value private conversations with our patients. When a patient records a visit, either covertly or overtly, most physicians pause and have some emotional reaction (either positive or negative). Some welcome the ability to communicate accurately to a wider audience, while others believe the act of recording violates an interpersonal bond. In this month’s issue, Dr. Adams discusses the legal and ethical ramifications when a patient records our clinical interactions. She offers an excellent analysis and practical risk management strategies. Personally, I follow my wife’s dictum to act as if I am always on camera.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Patients and physicians were collectively horrified last year when news broke of a Virginia man who recorded conversations between his gastroenterologist, his anesthesiologist, and other endoscopy unit staff, including a number of disparaging remarks about the patient, while sedated for his colonoscopy. Among other objectionable comments, providers mocked the patient for being demanding in the preprocedure area and for the amount of sedation he required, made comments implying that he had syphilis or tuberculosis, and discussed avoiding the patient following the procedure via an urgent “fake page.”1 The patient sued, resulting in a $500,000 judgment against the anesthesiologist for defamation and malpractice, including punitive damages. Although this case clearly represents an extreme example of unprofessional behavior, it also raises thought-provoking questions regarding the evolving relationship between patients and their physicians, as well as the legal and ethical implications of covert recording that deserve further discussion.
This article reviews the current state of knowledge regarding the frequency of and motivation for covert patient recording of medical encounters, and the legal and ethical principles informing this area. It concludes by proposing several strategies gastroenterologists can use to mitigate risk of liability while also preserving the patient-physician relationship and upholding professional autonomy.
Weighing the benefits and harms of patient electronic recording
Patient recording of medical encounters, whether covert or overt, presents both benefits and risks of harm. Theoretically, recording medical encounters could assist patients in remembering and/or better understanding recommendations provided by their physicians. It may also secondarily improve patient compliance and overall engagement in medical care and help patients accurately communicate recommendations to family members and other caregivers who are not immediately available during the clinical encounter. Patients may also view these recordings as a mechanism for empowerment, allowing them to shift the power dynamic between patient and provider.2,3 However, there is also the potential for recorded comments to be taken out of context or misinterpreted, leading to confusion on the part of the patient or family. Overt recording of medical encounters also may alter physician decision-making, leading to more aggressive testing and expense for the patient and the healthcare system. Even worse, covert recording of medical encounters (if discovered) may irreparably harm the physician-patient relationship by introducing distrust and causing the physician to take a more defensive posture in subsequent dealings with a given patient.
Recent research has shed new light on the potential frequency of patient covert recording of medical encounters, suggesting that it is alarmingly common. In a mixed-methods study of 130 patients in the United Kingdom who were recruited via radio and social media, 15% of respondents indicated that they had secretly recorded a clinical encounter, and an additional 11% personally knew someone who had covertly recorded.4 Those who reported having covertly recorded were significantly more likely to be less educated and male than those who had not. An additional 35% of respondents indicated that they would consider covertly recording a clinical encounter in the future. Although the generalizability of these results may be challenged based on the potential for sampling bias, the results suggest a shifting paradigm in the way in which patients view the physician-patient relationship and a fundamental breakdown in communication and erosion of trust.
The underlying motivations for patient recording of medical encounters are complex and multifaceted. These recordings seem to be a relatively new phenomenon and one that elicits strong reactions, positive and negative, on the part of patients, physicians, and society.2 Qualitative studies reveal that, whether covertly or overtly recording, most patients are driven by a common desire to replay, relisten, and/or share the recording with family, friends, and other caregivers.4 Indeed, the patient involved in the previously mentioned litigation purportedly intended to record the postcolonoscopy discharge instructions from his gastroenterologist, only to later discover much more. Patients who record covertly report being motivated by a fear of being denied permission to record or by prior experiences of poor quality care and the prospect of gathering verifiable evidence to support their experience. In contrast, patients who ask permission to record seem to be motivated primarily by a desire to preserve or enhance the physician-patient relationship.4 These insights are valuable in that they allow clinicians to view medical encounters from the perspective of patients, understand the power-dynamics at play, and ultimately, use this information to enlighten future care.
Legal guidance: “One-party” versus “all-party” consent
Although the prospect of covert patient recording may be unsettling to physicians, is it illegal? Because of a paucity of legal precedent in this area, the legal landscape is rather murky. Through the provisions of the Electronic Communications Privacy Act, federal law prohibits the interception and disclosure of wire, oral, or electronic communications without specific consent of at least one party to the conversation.5 This so-called “one-party” consent standard affords a baseline level of legal protection. A handful of states offer additional protection under state law by requiring all parties to the conversation to consent to the recording (so-called “all-party” consent). Virginia, where the audio recording of the previously mentioned colonoscopy took place, is a “one-party” consent state.6 In contrast, states such as California and Florida have adopted an “all-party” consent rule.7,8
However, uncertainty remains. For instance, if medical providers have a conversation in the same room as a sedated patient during a medical procedure on that patient, is the patient a “party” to the conversation? Furthermore, can such a conversation be considered private when held in front of a patient during a medical procedure? Is the patient in such a scenario “eavesdropping”? Given a lack of legal precedent in the form of case law and given the unique features of each clinical scenario, this is likely to remain an area of significant legal ambiguity. Although the possibility of covert patient recording may be unnerving for providers, the reality is that, in most cases, it is likely legally permissible.
Ethical principles: Navigating an evolving physician-patient relationship
The relationship between physician and patient, a core aspect of medical ethics, has evolved markedly over time. This relationship was historically paternalistic: The patient was seen to be dependent on the physician’s professional authority in determining the appropriateness of care, and patient’s preferences were seen as secondary to physician judgment. In recent years, however, the physician-patient relationship has evolved toward one privileging patient-centered care and shared decision-making based on a patient’s unique values, beliefs, and preferences.9,10
Concomitantly, the public’s view of doctoring has transitioned from “unquestioning acceptance of physician authority to a more ‘consumerist’ view accompanied by a questioning and bargaining approach to medicine, physicians and the medical encounter,” according to a 2001 study published in the Journal of Health and Social Behavior.11 In this context, many patients, attorneys, bioethicists, and patient advocates see patient recording of medical encounters as a legitimate check on the health care system, ensuring transparency and honesty and empowering patients to become more active participants in their medical care.2
Policy responses
Although recognition of patient recording of medical encounters has been growing, there have been few direct policy responses to date. One notable example was an effort by Wisconsin legislators in 2015 to mandate that any place where surgery is performed (including hospitals, ambulatory surgical centers, and other sites) offer patients the option to have their procedure videotaped and audiotaped.12 The proposed legislation was written broadly enough to include locations where gastroenterologists perform routine endoscopic procedures under sedation. Recordings would have been treated as part of the patient’s health care record and been admissible as evidence in subsequent legal proceedings relating to the medical care provided. Although this bill ultimately failed to pass pursuant to a Joint Resolution in the Wisconsin Senate in April 2016, it is a poignant example of the possible policy actions that may govern this area in the future.
In 2012, the National Institute for Health and Clinical Excellence, England, issued a guidance document focused on improving the patient care experience, which recommends that clinicians routinely ask patients if they would like to take notes and/or record the clinical encounter.13 Although not explicitly referencing covert recording, this guidance effectively aims to promote increased transparency by openly encouraging recording as a component of optimal medical practice. Although the American Medical Association Code of Ethics offers guidance regarding physicians recording patient encounters, it does not comment on patient-initiated recordings.14
Risk management strategies for gastroenterology providers
So, how can gastroenterologists and other providers protect themselves in a world of covert recording, while also preserving their relationships with patients and optimizing medical care? First, despite their harried days and varied responsibilities, gastroenterologists must recognize the possibility of covert recording and seek to maintain professionalism in all clinical environments, whether in an examination room or in an endoscopy suite with a sedated patient. Physicians set the tone for the entire team and also have an obligation to intervene if other members of the medical team are not adhering to professional standards. Although physicians and other medical providers often use cynical and derogatory humor as a coping mechanism given the heavy workload and amplified stressors of the clinical environment, it is important to be mindful of how such comments are perceived by patient bystanders.15,16 Although achieving a robust therapeutic alliance with a patient can take months, this trust can be easily broken by a single flippant remark by the physician.
Second, rather than assuming a defensive posture driven by fear of medical liability, it is vital for gastroenterologists to directly confront situations in which covert recording is suspected while also preserving the physician-patient relationship. In fact, by openly encouraging patient recording as a matter of routine practice, gastroenterologists can promote an environment of trust and transparency and bolster the therapeutic alliance between patient and provider.15 This approach also encourages providers to hone their communication skills and ensure they are communicating essential medical information clearly and succinctly and conveying medical nuance where appropriate. For example, in a clinic setting, a patient with inflammatory bowel disease who records might better remember or understand the risks and benefits of various treatment strategies and the common side effects of medications such as azathioprine and biologics. Patients recording medical encounters in the endoscopy suite might better recall postprocedure instructions, including recommended follow-up intervals and risks of postendoscopy complications. In this era of shared decision-making and patient-centered care, optimizing both physician delivery and patient understanding and recall of essential medical information is of critical importance.
Finally, although adoption of the above practices would serve the dual goals of enhancing patient-provider communication and mitigating legal risk, certain system interventions may further minimize the risk of covert recording. For instance, endoscopy units can store patients’ personal effects, including electronic devices, in a locker outside the endoscopy room rather than on the gurney. Retrieving patient belongings before postprocedure instructions are delivered would protect the patient’s ability to record this advice for future recall.
Conclusions
Recording by patients of clinical encounters, whether covert or overt, has become increasingly common as a result of the digital revolution. These recordings most often represent an attempt by the patient to gain more information relevant to their medical care. Rather than being threatened by this new reality, gastroenterologists should consider embracing this practice as an opportunity to enhance effective communication with patients, encourage shared decision making, and deliver truly patient-centered care.
Acknowledgments
This article is intended as general commentary and should not be interpreted as legal advice applicable to individual circumstances. Do not act or rely on information contained in this article without first seeking the advice of a personal attorney.
References
1. Washington Post. (2015 June 24). Audio: Anesthesiologist trashes sedated patient. Available at: https://youtu.be/Kar52idHgho. Accessed May 15, 2016.
2. Tsulukidze, M., Grande, S.W., Thompson, R., et al. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10:e0125824.
3. Rodriguez, M., Morrow, J., Selfi, A. Ethical implications of patients and families secretly recording conversations with physicians. JAMA. 2015;313(16):1615-6.
4. Elwyn, G., Barr, P.J., Grande, S.W. Patients recording clinical encounters: a path to empowerment? Assessment by mixed methods. BMJ Open. 2015;5(8):e008566.
5. 18 U.S.C. §2511(2)(d).
6. VA Code §19.2-62.
7. Cal. Penal Code §632.
8. Fla. Stat. Ann. §934.03(3)(d).
9. Truog, R.D. Patients and doctors: the evolution of a relationship. N Engl J Med. 2012;366:581-5.
10. Barry, M.J., Edgman-Levitan, S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366:780-1.
11. Pescosolido, B.A., Tuch, S.A., Martin, J.K. The profession of medicine and the public: examining Americans’ changing confidence in physician authority from the beginning of the ‘health care crisis’ to the era of health care reform. J Health Social Behavior. 2001;42(1):1-16.
12. Wisconsin Assembly Bill 255. (2015). Available at: https://docs.legis.wisconsin.gov/2015/proposals/ab255. Accessed June 29, 2016.
13. National Institute for Health and Clinical Excellence. (2012 Feb). Patient experience in adult NHS services: improving the experience of care for people using adult NHS service. Available at: https://www.nice.org.uk/guidance/cg138/chapter/1-guidance. Accessed June 29, 2016.
14. American Medical Association Code of Medical Ethics, Opinion 5.045 - Filming Patients in Health Care Settings. Updated June 2006. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5045.page? Accessed June 29, 2016.
15. Aultman, J.M. When humor in the hospital is no laughing matter. J Clin Ethics. 2009;20:227-35.
15. Sobel, R.K. Does laughter make good medicine? N Engl J Med. 2006;354:1114-5.
Dr. Adams is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor, an investigator with the VA Center for Clinical Management Research, a staff physician in the VA Ann Arbor Healthcare System, and a member of the Institute for Healthcare Policy and Innovation, Ann Arbor. She has no conflicts of interest.
Last year, while sedated for colonoscopy, a patient covertly recorded conversations between endoscopy staff and providers. Comments about the patient were egregious and resulted in loss of employment for those involved and a large financial settlement. The reality of today’s world is that we all are subject to constant (real or potential) surveillance. Nothing is private and nothing recorded is temporary, yet physicians value private conversations with our patients. When a patient records a visit, either covertly or overtly, most physicians pause and have some emotional reaction (either positive or negative). Some welcome the ability to communicate accurately to a wider audience, while others believe the act of recording violates an interpersonal bond. In this month’s issue, Dr. Adams discusses the legal and ethical ramifications when a patient records our clinical interactions. She offers an excellent analysis and practical risk management strategies. Personally, I follow my wife’s dictum to act as if I am always on camera.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Patients and physicians were collectively horrified last year when news broke of a Virginia man who recorded conversations between his gastroenterologist, his anesthesiologist, and other endoscopy unit staff, including a number of disparaging remarks about the patient, while sedated for his colonoscopy. Among other objectionable comments, providers mocked the patient for being demanding in the preprocedure area and for the amount of sedation he required, made comments implying that he had syphilis or tuberculosis, and discussed avoiding the patient following the procedure via an urgent “fake page.”1 The patient sued, resulting in a $500,000 judgment against the anesthesiologist for defamation and malpractice, including punitive damages. Although this case clearly represents an extreme example of unprofessional behavior, it also raises thought-provoking questions regarding the evolving relationship between patients and their physicians, as well as the legal and ethical implications of covert recording that deserve further discussion.
This article reviews the current state of knowledge regarding the frequency of and motivation for covert patient recording of medical encounters, and the legal and ethical principles informing this area. It concludes by proposing several strategies gastroenterologists can use to mitigate risk of liability while also preserving the patient-physician relationship and upholding professional autonomy.
Weighing the benefits and harms of patient electronic recording
Patient recording of medical encounters, whether covert or overt, presents both benefits and risks of harm. Theoretically, recording medical encounters could assist patients in remembering and/or better understanding recommendations provided by their physicians. It may also secondarily improve patient compliance and overall engagement in medical care and help patients accurately communicate recommendations to family members and other caregivers who are not immediately available during the clinical encounter. Patients may also view these recordings as a mechanism for empowerment, allowing them to shift the power dynamic between patient and provider.2,3 However, there is also the potential for recorded comments to be taken out of context or misinterpreted, leading to confusion on the part of the patient or family. Overt recording of medical encounters also may alter physician decision-making, leading to more aggressive testing and expense for the patient and the healthcare system. Even worse, covert recording of medical encounters (if discovered) may irreparably harm the physician-patient relationship by introducing distrust and causing the physician to take a more defensive posture in subsequent dealings with a given patient.
Recent research has shed new light on the potential frequency of patient covert recording of medical encounters, suggesting that it is alarmingly common. In a mixed-methods study of 130 patients in the United Kingdom who were recruited via radio and social media, 15% of respondents indicated that they had secretly recorded a clinical encounter, and an additional 11% personally knew someone who had covertly recorded.4 Those who reported having covertly recorded were significantly more likely to be less educated and male than those who had not. An additional 35% of respondents indicated that they would consider covertly recording a clinical encounter in the future. Although the generalizability of these results may be challenged based on the potential for sampling bias, the results suggest a shifting paradigm in the way in which patients view the physician-patient relationship and a fundamental breakdown in communication and erosion of trust.
The underlying motivations for patient recording of medical encounters are complex and multifaceted. These recordings seem to be a relatively new phenomenon and one that elicits strong reactions, positive and negative, on the part of patients, physicians, and society.2 Qualitative studies reveal that, whether covertly or overtly recording, most patients are driven by a common desire to replay, relisten, and/or share the recording with family, friends, and other caregivers.4 Indeed, the patient involved in the previously mentioned litigation purportedly intended to record the postcolonoscopy discharge instructions from his gastroenterologist, only to later discover much more. Patients who record covertly report being motivated by a fear of being denied permission to record or by prior experiences of poor quality care and the prospect of gathering verifiable evidence to support their experience. In contrast, patients who ask permission to record seem to be motivated primarily by a desire to preserve or enhance the physician-patient relationship.4 These insights are valuable in that they allow clinicians to view medical encounters from the perspective of patients, understand the power-dynamics at play, and ultimately, use this information to enlighten future care.
Legal guidance: “One-party” versus “all-party” consent
Although the prospect of covert patient recording may be unsettling to physicians, is it illegal? Because of a paucity of legal precedent in this area, the legal landscape is rather murky. Through the provisions of the Electronic Communications Privacy Act, federal law prohibits the interception and disclosure of wire, oral, or electronic communications without specific consent of at least one party to the conversation.5 This so-called “one-party” consent standard affords a baseline level of legal protection. A handful of states offer additional protection under state law by requiring all parties to the conversation to consent to the recording (so-called “all-party” consent). Virginia, where the audio recording of the previously mentioned colonoscopy took place, is a “one-party” consent state.6 In contrast, states such as California and Florida have adopted an “all-party” consent rule.7,8
However, uncertainty remains. For instance, if medical providers have a conversation in the same room as a sedated patient during a medical procedure on that patient, is the patient a “party” to the conversation? Furthermore, can such a conversation be considered private when held in front of a patient during a medical procedure? Is the patient in such a scenario “eavesdropping”? Given a lack of legal precedent in the form of case law and given the unique features of each clinical scenario, this is likely to remain an area of significant legal ambiguity. Although the possibility of covert patient recording may be unnerving for providers, the reality is that, in most cases, it is likely legally permissible.
Ethical principles: Navigating an evolving physician-patient relationship
The relationship between physician and patient, a core aspect of medical ethics, has evolved markedly over time. This relationship was historically paternalistic: The patient was seen to be dependent on the physician’s professional authority in determining the appropriateness of care, and patient’s preferences were seen as secondary to physician judgment. In recent years, however, the physician-patient relationship has evolved toward one privileging patient-centered care and shared decision-making based on a patient’s unique values, beliefs, and preferences.9,10
Concomitantly, the public’s view of doctoring has transitioned from “unquestioning acceptance of physician authority to a more ‘consumerist’ view accompanied by a questioning and bargaining approach to medicine, physicians and the medical encounter,” according to a 2001 study published in the Journal of Health and Social Behavior.11 In this context, many patients, attorneys, bioethicists, and patient advocates see patient recording of medical encounters as a legitimate check on the health care system, ensuring transparency and honesty and empowering patients to become more active participants in their medical care.2
Policy responses
Although recognition of patient recording of medical encounters has been growing, there have been few direct policy responses to date. One notable example was an effort by Wisconsin legislators in 2015 to mandate that any place where surgery is performed (including hospitals, ambulatory surgical centers, and other sites) offer patients the option to have their procedure videotaped and audiotaped.12 The proposed legislation was written broadly enough to include locations where gastroenterologists perform routine endoscopic procedures under sedation. Recordings would have been treated as part of the patient’s health care record and been admissible as evidence in subsequent legal proceedings relating to the medical care provided. Although this bill ultimately failed to pass pursuant to a Joint Resolution in the Wisconsin Senate in April 2016, it is a poignant example of the possible policy actions that may govern this area in the future.
In 2012, the National Institute for Health and Clinical Excellence, England, issued a guidance document focused on improving the patient care experience, which recommends that clinicians routinely ask patients if they would like to take notes and/or record the clinical encounter.13 Although not explicitly referencing covert recording, this guidance effectively aims to promote increased transparency by openly encouraging recording as a component of optimal medical practice. Although the American Medical Association Code of Ethics offers guidance regarding physicians recording patient encounters, it does not comment on patient-initiated recordings.14
Risk management strategies for gastroenterology providers
So, how can gastroenterologists and other providers protect themselves in a world of covert recording, while also preserving their relationships with patients and optimizing medical care? First, despite their harried days and varied responsibilities, gastroenterologists must recognize the possibility of covert recording and seek to maintain professionalism in all clinical environments, whether in an examination room or in an endoscopy suite with a sedated patient. Physicians set the tone for the entire team and also have an obligation to intervene if other members of the medical team are not adhering to professional standards. Although physicians and other medical providers often use cynical and derogatory humor as a coping mechanism given the heavy workload and amplified stressors of the clinical environment, it is important to be mindful of how such comments are perceived by patient bystanders.15,16 Although achieving a robust therapeutic alliance with a patient can take months, this trust can be easily broken by a single flippant remark by the physician.
Second, rather than assuming a defensive posture driven by fear of medical liability, it is vital for gastroenterologists to directly confront situations in which covert recording is suspected while also preserving the physician-patient relationship. In fact, by openly encouraging patient recording as a matter of routine practice, gastroenterologists can promote an environment of trust and transparency and bolster the therapeutic alliance between patient and provider.15 This approach also encourages providers to hone their communication skills and ensure they are communicating essential medical information clearly and succinctly and conveying medical nuance where appropriate. For example, in a clinic setting, a patient with inflammatory bowel disease who records might better remember or understand the risks and benefits of various treatment strategies and the common side effects of medications such as azathioprine and biologics. Patients recording medical encounters in the endoscopy suite might better recall postprocedure instructions, including recommended follow-up intervals and risks of postendoscopy complications. In this era of shared decision-making and patient-centered care, optimizing both physician delivery and patient understanding and recall of essential medical information is of critical importance.
Finally, although adoption of the above practices would serve the dual goals of enhancing patient-provider communication and mitigating legal risk, certain system interventions may further minimize the risk of covert recording. For instance, endoscopy units can store patients’ personal effects, including electronic devices, in a locker outside the endoscopy room rather than on the gurney. Retrieving patient belongings before postprocedure instructions are delivered would protect the patient’s ability to record this advice for future recall.
Conclusions
Recording by patients of clinical encounters, whether covert or overt, has become increasingly common as a result of the digital revolution. These recordings most often represent an attempt by the patient to gain more information relevant to their medical care. Rather than being threatened by this new reality, gastroenterologists should consider embracing this practice as an opportunity to enhance effective communication with patients, encourage shared decision making, and deliver truly patient-centered care.
Acknowledgments
This article is intended as general commentary and should not be interpreted as legal advice applicable to individual circumstances. Do not act or rely on information contained in this article without first seeking the advice of a personal attorney.
References
1. Washington Post. (2015 June 24). Audio: Anesthesiologist trashes sedated patient. Available at: https://youtu.be/Kar52idHgho. Accessed May 15, 2016.
2. Tsulukidze, M., Grande, S.W., Thompson, R., et al. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10:e0125824.
3. Rodriguez, M., Morrow, J., Selfi, A. Ethical implications of patients and families secretly recording conversations with physicians. JAMA. 2015;313(16):1615-6.
4. Elwyn, G., Barr, P.J., Grande, S.W. Patients recording clinical encounters: a path to empowerment? Assessment by mixed methods. BMJ Open. 2015;5(8):e008566.
5. 18 U.S.C. §2511(2)(d).
6. VA Code §19.2-62.
7. Cal. Penal Code §632.
8. Fla. Stat. Ann. §934.03(3)(d).
9. Truog, R.D. Patients and doctors: the evolution of a relationship. N Engl J Med. 2012;366:581-5.
10. Barry, M.J., Edgman-Levitan, S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366:780-1.
11. Pescosolido, B.A., Tuch, S.A., Martin, J.K. The profession of medicine and the public: examining Americans’ changing confidence in physician authority from the beginning of the ‘health care crisis’ to the era of health care reform. J Health Social Behavior. 2001;42(1):1-16.
12. Wisconsin Assembly Bill 255. (2015). Available at: https://docs.legis.wisconsin.gov/2015/proposals/ab255. Accessed June 29, 2016.
13. National Institute for Health and Clinical Excellence. (2012 Feb). Patient experience in adult NHS services: improving the experience of care for people using adult NHS service. Available at: https://www.nice.org.uk/guidance/cg138/chapter/1-guidance. Accessed June 29, 2016.
14. American Medical Association Code of Medical Ethics, Opinion 5.045 - Filming Patients in Health Care Settings. Updated June 2006. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5045.page? Accessed June 29, 2016.
15. Aultman, J.M. When humor in the hospital is no laughing matter. J Clin Ethics. 2009;20:227-35.
15. Sobel, R.K. Does laughter make good medicine? N Engl J Med. 2006;354:1114-5.
Dr. Adams is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor, an investigator with the VA Center for Clinical Management Research, a staff physician in the VA Ann Arbor Healthcare System, and a member of the Institute for Healthcare Policy and Innovation, Ann Arbor. She has no conflicts of interest.
Last year, while sedated for colonoscopy, a patient covertly recorded conversations between endoscopy staff and providers. Comments about the patient were egregious and resulted in loss of employment for those involved and a large financial settlement. The reality of today’s world is that we all are subject to constant (real or potential) surveillance. Nothing is private and nothing recorded is temporary, yet physicians value private conversations with our patients. When a patient records a visit, either covertly or overtly, most physicians pause and have some emotional reaction (either positive or negative). Some welcome the ability to communicate accurately to a wider audience, while others believe the act of recording violates an interpersonal bond. In this month’s issue, Dr. Adams discusses the legal and ethical ramifications when a patient records our clinical interactions. She offers an excellent analysis and practical risk management strategies. Personally, I follow my wife’s dictum to act as if I am always on camera.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Patients and physicians were collectively horrified last year when news broke of a Virginia man who recorded conversations between his gastroenterologist, his anesthesiologist, and other endoscopy unit staff, including a number of disparaging remarks about the patient, while sedated for his colonoscopy. Among other objectionable comments, providers mocked the patient for being demanding in the preprocedure area and for the amount of sedation he required, made comments implying that he had syphilis or tuberculosis, and discussed avoiding the patient following the procedure via an urgent “fake page.”1 The patient sued, resulting in a $500,000 judgment against the anesthesiologist for defamation and malpractice, including punitive damages. Although this case clearly represents an extreme example of unprofessional behavior, it also raises thought-provoking questions regarding the evolving relationship between patients and their physicians, as well as the legal and ethical implications of covert recording that deserve further discussion.
This article reviews the current state of knowledge regarding the frequency of and motivation for covert patient recording of medical encounters, and the legal and ethical principles informing this area. It concludes by proposing several strategies gastroenterologists can use to mitigate risk of liability while also preserving the patient-physician relationship and upholding professional autonomy.
Weighing the benefits and harms of patient electronic recording
Patient recording of medical encounters, whether covert or overt, presents both benefits and risks of harm. Theoretically, recording medical encounters could assist patients in remembering and/or better understanding recommendations provided by their physicians. It may also secondarily improve patient compliance and overall engagement in medical care and help patients accurately communicate recommendations to family members and other caregivers who are not immediately available during the clinical encounter. Patients may also view these recordings as a mechanism for empowerment, allowing them to shift the power dynamic between patient and provider.2,3 However, there is also the potential for recorded comments to be taken out of context or misinterpreted, leading to confusion on the part of the patient or family. Overt recording of medical encounters also may alter physician decision-making, leading to more aggressive testing and expense for the patient and the healthcare system. Even worse, covert recording of medical encounters (if discovered) may irreparably harm the physician-patient relationship by introducing distrust and causing the physician to take a more defensive posture in subsequent dealings with a given patient.
Recent research has shed new light on the potential frequency of patient covert recording of medical encounters, suggesting that it is alarmingly common. In a mixed-methods study of 130 patients in the United Kingdom who were recruited via radio and social media, 15% of respondents indicated that they had secretly recorded a clinical encounter, and an additional 11% personally knew someone who had covertly recorded.4 Those who reported having covertly recorded were significantly more likely to be less educated and male than those who had not. An additional 35% of respondents indicated that they would consider covertly recording a clinical encounter in the future. Although the generalizability of these results may be challenged based on the potential for sampling bias, the results suggest a shifting paradigm in the way in which patients view the physician-patient relationship and a fundamental breakdown in communication and erosion of trust.
The underlying motivations for patient recording of medical encounters are complex and multifaceted. These recordings seem to be a relatively new phenomenon and one that elicits strong reactions, positive and negative, on the part of patients, physicians, and society.2 Qualitative studies reveal that, whether covertly or overtly recording, most patients are driven by a common desire to replay, relisten, and/or share the recording with family, friends, and other caregivers.4 Indeed, the patient involved in the previously mentioned litigation purportedly intended to record the postcolonoscopy discharge instructions from his gastroenterologist, only to later discover much more. Patients who record covertly report being motivated by a fear of being denied permission to record or by prior experiences of poor quality care and the prospect of gathering verifiable evidence to support their experience. In contrast, patients who ask permission to record seem to be motivated primarily by a desire to preserve or enhance the physician-patient relationship.4 These insights are valuable in that they allow clinicians to view medical encounters from the perspective of patients, understand the power-dynamics at play, and ultimately, use this information to enlighten future care.
Legal guidance: “One-party” versus “all-party” consent
Although the prospect of covert patient recording may be unsettling to physicians, is it illegal? Because of a paucity of legal precedent in this area, the legal landscape is rather murky. Through the provisions of the Electronic Communications Privacy Act, federal law prohibits the interception and disclosure of wire, oral, or electronic communications without specific consent of at least one party to the conversation.5 This so-called “one-party” consent standard affords a baseline level of legal protection. A handful of states offer additional protection under state law by requiring all parties to the conversation to consent to the recording (so-called “all-party” consent). Virginia, where the audio recording of the previously mentioned colonoscopy took place, is a “one-party” consent state.6 In contrast, states such as California and Florida have adopted an “all-party” consent rule.7,8
However, uncertainty remains. For instance, if medical providers have a conversation in the same room as a sedated patient during a medical procedure on that patient, is the patient a “party” to the conversation? Furthermore, can such a conversation be considered private when held in front of a patient during a medical procedure? Is the patient in such a scenario “eavesdropping”? Given a lack of legal precedent in the form of case law and given the unique features of each clinical scenario, this is likely to remain an area of significant legal ambiguity. Although the possibility of covert patient recording may be unnerving for providers, the reality is that, in most cases, it is likely legally permissible.
Ethical principles: Navigating an evolving physician-patient relationship
The relationship between physician and patient, a core aspect of medical ethics, has evolved markedly over time. This relationship was historically paternalistic: The patient was seen to be dependent on the physician’s professional authority in determining the appropriateness of care, and patient’s preferences were seen as secondary to physician judgment. In recent years, however, the physician-patient relationship has evolved toward one privileging patient-centered care and shared decision-making based on a patient’s unique values, beliefs, and preferences.9,10
Concomitantly, the public’s view of doctoring has transitioned from “unquestioning acceptance of physician authority to a more ‘consumerist’ view accompanied by a questioning and bargaining approach to medicine, physicians and the medical encounter,” according to a 2001 study published in the Journal of Health and Social Behavior.11 In this context, many patients, attorneys, bioethicists, and patient advocates see patient recording of medical encounters as a legitimate check on the health care system, ensuring transparency and honesty and empowering patients to become more active participants in their medical care.2
Policy responses
Although recognition of patient recording of medical encounters has been growing, there have been few direct policy responses to date. One notable example was an effort by Wisconsin legislators in 2015 to mandate that any place where surgery is performed (including hospitals, ambulatory surgical centers, and other sites) offer patients the option to have their procedure videotaped and audiotaped.12 The proposed legislation was written broadly enough to include locations where gastroenterologists perform routine endoscopic procedures under sedation. Recordings would have been treated as part of the patient’s health care record and been admissible as evidence in subsequent legal proceedings relating to the medical care provided. Although this bill ultimately failed to pass pursuant to a Joint Resolution in the Wisconsin Senate in April 2016, it is a poignant example of the possible policy actions that may govern this area in the future.
In 2012, the National Institute for Health and Clinical Excellence, England, issued a guidance document focused on improving the patient care experience, which recommends that clinicians routinely ask patients if they would like to take notes and/or record the clinical encounter.13 Although not explicitly referencing covert recording, this guidance effectively aims to promote increased transparency by openly encouraging recording as a component of optimal medical practice. Although the American Medical Association Code of Ethics offers guidance regarding physicians recording patient encounters, it does not comment on patient-initiated recordings.14
Risk management strategies for gastroenterology providers
So, how can gastroenterologists and other providers protect themselves in a world of covert recording, while also preserving their relationships with patients and optimizing medical care? First, despite their harried days and varied responsibilities, gastroenterologists must recognize the possibility of covert recording and seek to maintain professionalism in all clinical environments, whether in an examination room or in an endoscopy suite with a sedated patient. Physicians set the tone for the entire team and also have an obligation to intervene if other members of the medical team are not adhering to professional standards. Although physicians and other medical providers often use cynical and derogatory humor as a coping mechanism given the heavy workload and amplified stressors of the clinical environment, it is important to be mindful of how such comments are perceived by patient bystanders.15,16 Although achieving a robust therapeutic alliance with a patient can take months, this trust can be easily broken by a single flippant remark by the physician.
Second, rather than assuming a defensive posture driven by fear of medical liability, it is vital for gastroenterologists to directly confront situations in which covert recording is suspected while also preserving the physician-patient relationship. In fact, by openly encouraging patient recording as a matter of routine practice, gastroenterologists can promote an environment of trust and transparency and bolster the therapeutic alliance between patient and provider.15 This approach also encourages providers to hone their communication skills and ensure they are communicating essential medical information clearly and succinctly and conveying medical nuance where appropriate. For example, in a clinic setting, a patient with inflammatory bowel disease who records might better remember or understand the risks and benefits of various treatment strategies and the common side effects of medications such as azathioprine and biologics. Patients recording medical encounters in the endoscopy suite might better recall postprocedure instructions, including recommended follow-up intervals and risks of postendoscopy complications. In this era of shared decision-making and patient-centered care, optimizing both physician delivery and patient understanding and recall of essential medical information is of critical importance.
Finally, although adoption of the above practices would serve the dual goals of enhancing patient-provider communication and mitigating legal risk, certain system interventions may further minimize the risk of covert recording. For instance, endoscopy units can store patients’ personal effects, including electronic devices, in a locker outside the endoscopy room rather than on the gurney. Retrieving patient belongings before postprocedure instructions are delivered would protect the patient’s ability to record this advice for future recall.
Conclusions
Recording by patients of clinical encounters, whether covert or overt, has become increasingly common as a result of the digital revolution. These recordings most often represent an attempt by the patient to gain more information relevant to their medical care. Rather than being threatened by this new reality, gastroenterologists should consider embracing this practice as an opportunity to enhance effective communication with patients, encourage shared decision making, and deliver truly patient-centered care.
Acknowledgments
This article is intended as general commentary and should not be interpreted as legal advice applicable to individual circumstances. Do not act or rely on information contained in this article without first seeking the advice of a personal attorney.
References
1. Washington Post. (2015 June 24). Audio: Anesthesiologist trashes sedated patient. Available at: https://youtu.be/Kar52idHgho. Accessed May 15, 2016.
2. Tsulukidze, M., Grande, S.W., Thompson, R., et al. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10:e0125824.
3. Rodriguez, M., Morrow, J., Selfi, A. Ethical implications of patients and families secretly recording conversations with physicians. JAMA. 2015;313(16):1615-6.
4. Elwyn, G., Barr, P.J., Grande, S.W. Patients recording clinical encounters: a path to empowerment? Assessment by mixed methods. BMJ Open. 2015;5(8):e008566.
5. 18 U.S.C. §2511(2)(d).
6. VA Code §19.2-62.
7. Cal. Penal Code §632.
8. Fla. Stat. Ann. §934.03(3)(d).
9. Truog, R.D. Patients and doctors: the evolution of a relationship. N Engl J Med. 2012;366:581-5.
10. Barry, M.J., Edgman-Levitan, S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366:780-1.
11. Pescosolido, B.A., Tuch, S.A., Martin, J.K. The profession of medicine and the public: examining Americans’ changing confidence in physician authority from the beginning of the ‘health care crisis’ to the era of health care reform. J Health Social Behavior. 2001;42(1):1-16.
12. Wisconsin Assembly Bill 255. (2015). Available at: https://docs.legis.wisconsin.gov/2015/proposals/ab255. Accessed June 29, 2016.
13. National Institute for Health and Clinical Excellence. (2012 Feb). Patient experience in adult NHS services: improving the experience of care for people using adult NHS service. Available at: https://www.nice.org.uk/guidance/cg138/chapter/1-guidance. Accessed June 29, 2016.
14. American Medical Association Code of Medical Ethics, Opinion 5.045 - Filming Patients in Health Care Settings. Updated June 2006. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion5045.page? Accessed June 29, 2016.
15. Aultman, J.M. When humor in the hospital is no laughing matter. J Clin Ethics. 2009;20:227-35.
15. Sobel, R.K. Does laughter make good medicine? N Engl J Med. 2006;354:1114-5.
Dr. Adams is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor, an investigator with the VA Center for Clinical Management Research, a staff physician in the VA Ann Arbor Healthcare System, and a member of the Institute for Healthcare Policy and Innovation, Ann Arbor. She has no conflicts of interest.