User login
Aneuploidy screening: Newer noninvasive test gains traction
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Should You Bypass Anticoagulant “Bridging” Before and After Surgery?
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
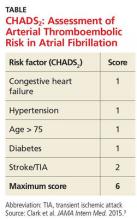
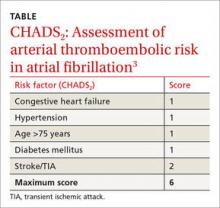
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
Should you bypass anticoagulant “bridging” before and after surgery?
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Sterile or Nonsterile Gloves for Minor Skin Excisions?
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
This Adjunct Medication Can Speed CAP Recovery
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
PRACTICE CHANGER
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild-to-moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
STRENGTH OF RECOMMENDATION
A: Based on a single good-quality randomized controlled trial (RCT) and meta-analysis.1
ILLUSTRATIVE CASE
A 75-year-old woman with hypertension and diabetes presents to the emergency department with shortness of breath, cough, and fever that she’s had for four days. On examination, her temperature is 38.2°C; heart rate, 110 beats/min; respiratory rate, 28 breaths/min; and O2 saturation, 91%. Rhonchi are heard in her right lower lung field; chest x-ray reveals infiltrate in her right lower lobe. The patient is admitted and started on IV antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than 1 million hospitalizations annually in the United States and is the eighth leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures (eg, IV fluids and antipyretics). Because the disease process involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified six small RCTs that evaluated the impact of corticosteroids on CAP recovery.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk for bias.
Subsequently, a 2012 meta-analysis of nine RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of eight moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY
Prednisone hastens clinical stabilization, cuts hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP who were admitted to one of seven tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible if they were 18 or older, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had a contraindication to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for seven days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on day 30.
The primary outcome was length of time to clinical stability (eg, at least 24 hours of stable vital signs). This composite endpoint required all of the following: temperature ≤ 37.8°C; heart rate ≤ 100 beats/min; spontaneous respiratory rate ≤ 24 breaths/min; systolic blood pressure ≥ 90 mm Hg (≥ 100 mm Hg for patients diagnosed with hypertension) without vasopressor support; mental status back to baseline; ability to take food by mouth; and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR], 2.5 to 3.4) compared to the placebo group at 4.4 days (IQR, 4 - 5; hazard ratio [HR], 1.33). Median time to hospital discharge was also shorter for the prednisone group (6 d vs 7 d; HR, 1.19), as was duration of IV antibiotic treatment (4 d vs 5 d; difference, –0.89 d).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio, 1.96).
WHAT’S NEW
This large, good-quality study reinforces previous evidence
This is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay. It also used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS
It’s unclear if steroids benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, although this was a large, well-designed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP.
Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the US.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION
Risk for adverse events
Treatment with prednisone increases risk for corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these effects appear to resolve quickly after treatment and do not impact the overall time to clinical stability.
REFERENCES
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. CDC. FastStats: Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed September 29, 2015.
3. CDC/National Center for Health Statistics. Top 10 leading causes of death: United States, 1999–2013. http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 29, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519-528.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(10):648-650.
Sterile or non-sterile gloves for minor skin excisions?
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
This adjunct medication can speed CAP recovery
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
It’s Time to Reconsider Early-morning Testosterone Tests
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
It’s time to reconsider early-morning testosterone tests
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
Copyright © 2015 Family Physicians Inquiries Network. All rights reserved.
Another Good Reason to Recommend Low-dose Aspirin
PRACTICE CHANGER
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk for this complication, as well as preterm birth and intrauterine growth restriction.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.1
ILLUSTRATIVE CASE
A 22-year-old G2P1 pregnant woman at 18 weeks’ gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk for preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at > 20 weeks’ gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4
The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors include previous preeclampsia, maternal age 40 or older, chronic medical conditions, and multifetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for prevention would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY
Aspirin lowers risk for preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized, placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good quality.
Most study participants were white and ages 20 to 33. Aspirin doses ranged from 60 to 150 mg/d; most studies used doses of 60 or 100 mg/d. Aspirin was initiated between 12 to 36 weeks’ gestation, with nine trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 for low-dose aspirin, compared to placebo. However, this finding was not statistically significant (P = .78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR, 0.86), a 20% risk reduction for IUGR (RR, 0.80), and a 24% risk reduction for preeclampsia (RR, 0.76). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by “small study effects” (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR, 1.02). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR, 0.92; P = .65). No differences were noted in the toddlers’ development at 18 months.
WHAT’S NEW
Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for the complication (Grade B).9
CAVEATS
Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend not to be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION
You need to determine which patients are at highest risk
The principle challenge lies in the identification of patients who are at high risk for preeclampsia and thus will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was at high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined as high risk women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: previous preeclampsia, multifetal gestation, chronic hypertension, diabetes, renal disease, or autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
REFERENCES
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005; 330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. 2nd ed. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659. 8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369: 1791-1798. 9. LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia [recommendation statement]. Ann Intern Med. 2014;161:819-826.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from The Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(5):301-303.
PRACTICE CHANGER
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk for this complication, as well as preterm birth and intrauterine growth restriction.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.1
ILLUSTRATIVE CASE
A 22-year-old G2P1 pregnant woman at 18 weeks’ gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk for preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at > 20 weeks’ gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4
The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors include previous preeclampsia, maternal age 40 or older, chronic medical conditions, and multifetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for prevention would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY
Aspirin lowers risk for preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized, placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good quality.
Most study participants were white and ages 20 to 33. Aspirin doses ranged from 60 to 150 mg/d; most studies used doses of 60 or 100 mg/d. Aspirin was initiated between 12 to 36 weeks’ gestation, with nine trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 for low-dose aspirin, compared to placebo. However, this finding was not statistically significant (P = .78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR, 0.86), a 20% risk reduction for IUGR (RR, 0.80), and a 24% risk reduction for preeclampsia (RR, 0.76). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by “small study effects” (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR, 1.02). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR, 0.92; P = .65). No differences were noted in the toddlers’ development at 18 months.
WHAT’S NEW
Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for the complication (Grade B).9
CAVEATS
Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend not to be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION
You need to determine which patients are at highest risk
The principle challenge lies in the identification of patients who are at high risk for preeclampsia and thus will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was at high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined as high risk women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: previous preeclampsia, multifetal gestation, chronic hypertension, diabetes, renal disease, or autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
REFERENCES
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005; 330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. 2nd ed. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659. 8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369: 1791-1798. 9. LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia [recommendation statement]. Ann Intern Med. 2014;161:819-826.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from The Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(5):301-303.
PRACTICE CHANGER
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk for this complication, as well as preterm birth and intrauterine growth restriction.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.1
ILLUSTRATIVE CASE
A 22-year-old G2P1 pregnant woman at 18 weeks’ gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk for preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at > 20 weeks’ gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4
The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors include previous preeclampsia, maternal age 40 or older, chronic medical conditions, and multifetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for prevention would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY
Aspirin lowers risk for preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized, placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good quality.
Most study participants were white and ages 20 to 33. Aspirin doses ranged from 60 to 150 mg/d; most studies used doses of 60 or 100 mg/d. Aspirin was initiated between 12 to 36 weeks’ gestation, with nine trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 for low-dose aspirin, compared to placebo. However, this finding was not statistically significant (P = .78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR, 0.86), a 20% risk reduction for IUGR (RR, 0.80), and a 24% risk reduction for preeclampsia (RR, 0.76). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by “small study effects” (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR, 1.02). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR, 0.92; P = .65). No differences were noted in the toddlers’ development at 18 months.
WHAT’S NEW
Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for the complication (Grade B).9
CAVEATS
Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend not to be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION
You need to determine which patients are at highest risk
The principle challenge lies in the identification of patients who are at high risk for preeclampsia and thus will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was at high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined as high risk women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: previous preeclampsia, multifetal gestation, chronic hypertension, diabetes, renal disease, or autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
REFERENCES
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005; 330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. 2nd ed. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659. 8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369: 1791-1798. 9. LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia [recommendation statement]. Ann Intern Med. 2014;161:819-826.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from The Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(5):301-303.