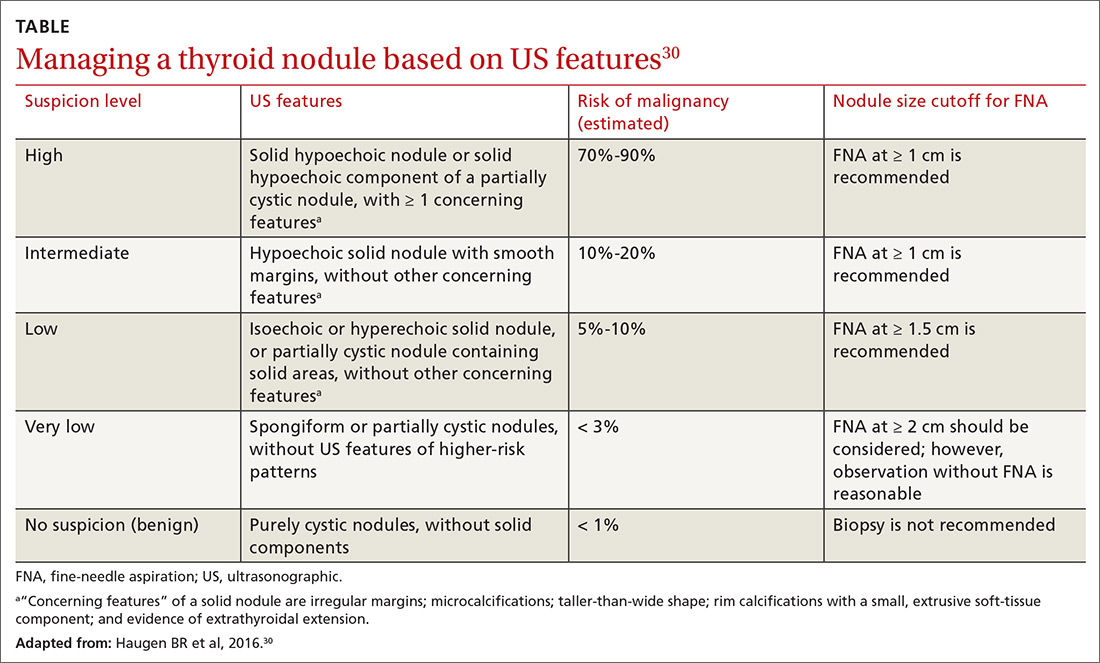
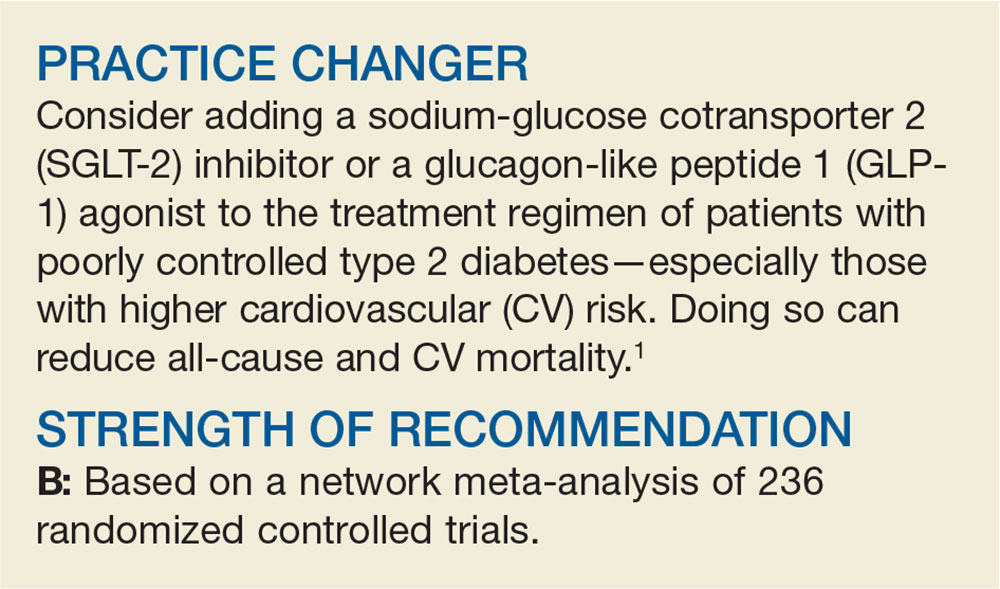
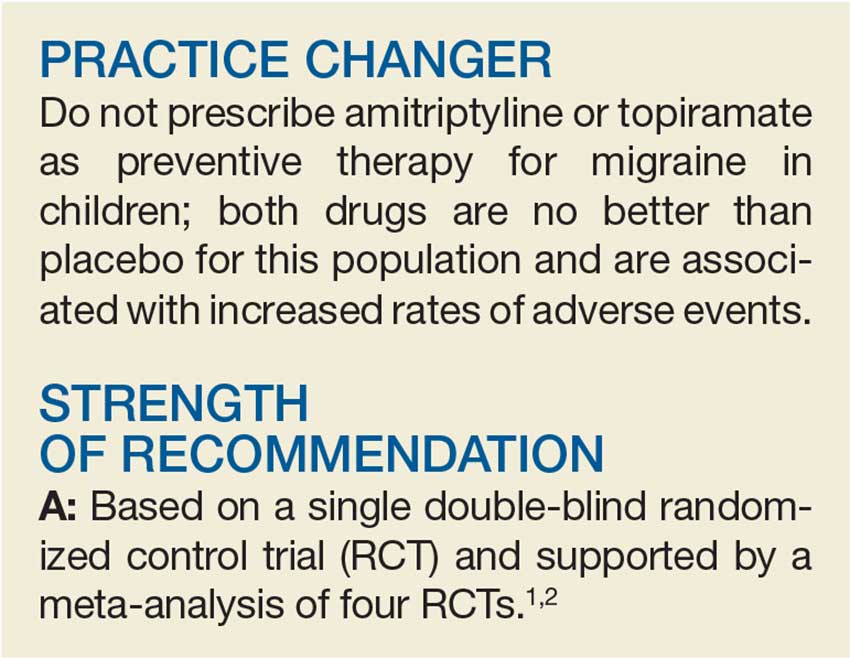
User login
Is there benefit to adding ezetimibe to a statin for the secondary prevention of CVD?
Evidence summary
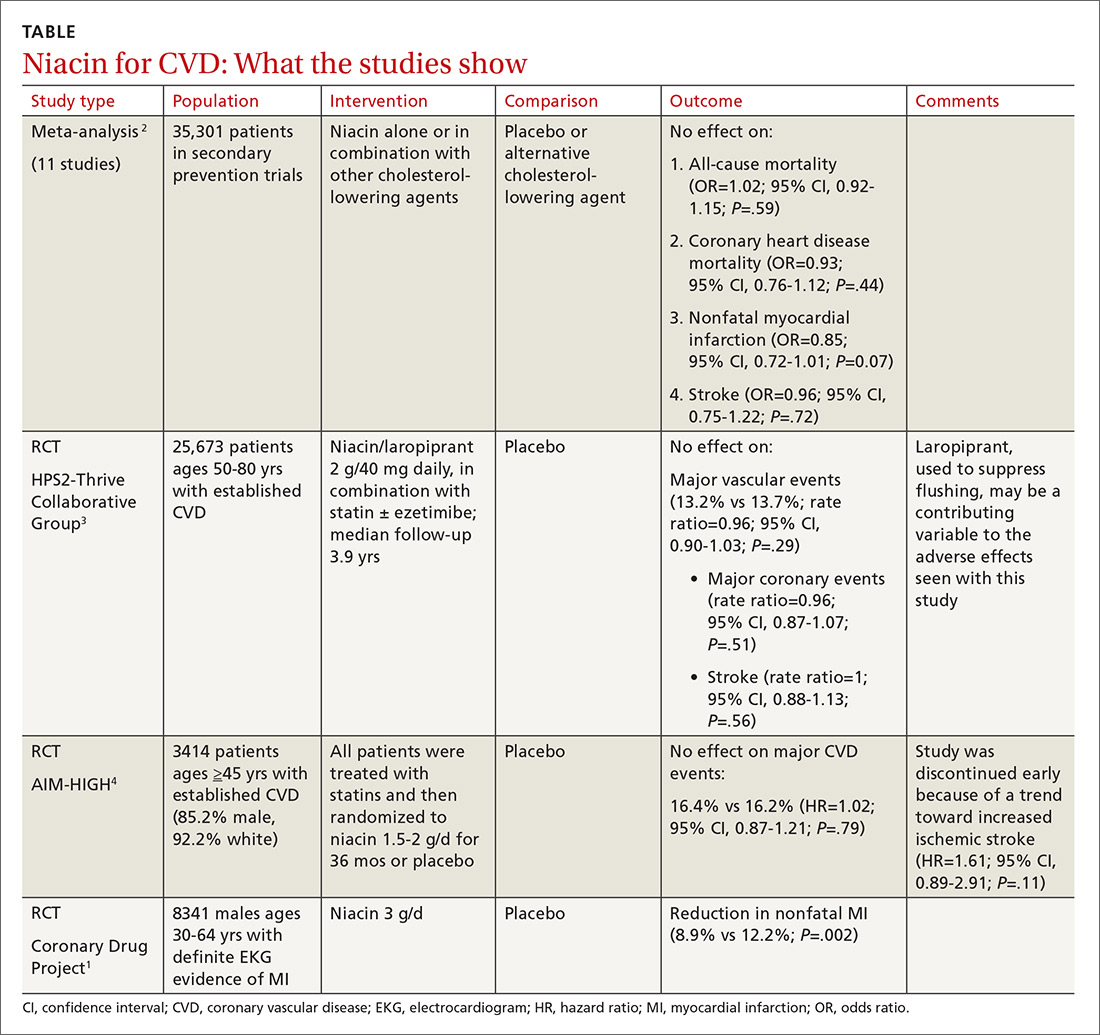
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
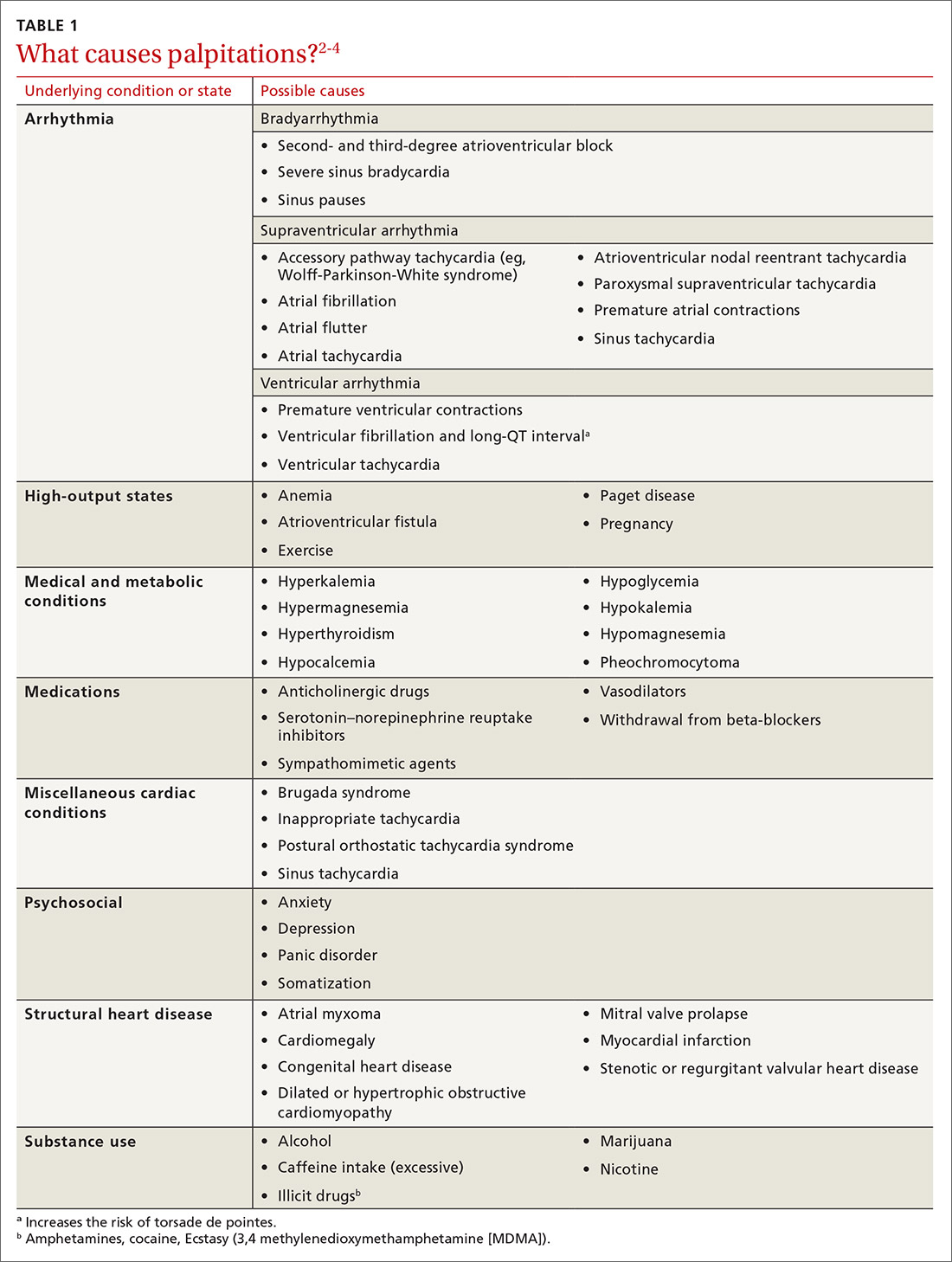
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.
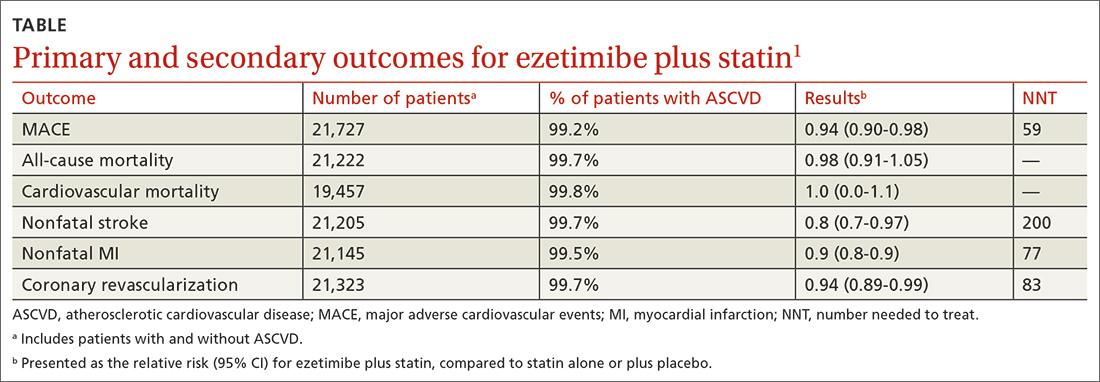
The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
EVIDENCE-BASED REVIEW:
YES. In patients with known cardio- vascular disease (CVD), ezetimibe with a statin decreases
In adults with atherosclerotic CVD (ASCVD), the combination of ezetimibe and a moderate-intensity statin (rosuvastatin 10 mg) was noninferior at decreasing cardiovascular death, major cardiovascular events, and nonfatal stroke, but was more tolerable, compared to a high-intensity statin (rosuvastatin 20 mg) alone (SOR, B; 1 RCT).
Tips and tools to help refine your approach to chest pain
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
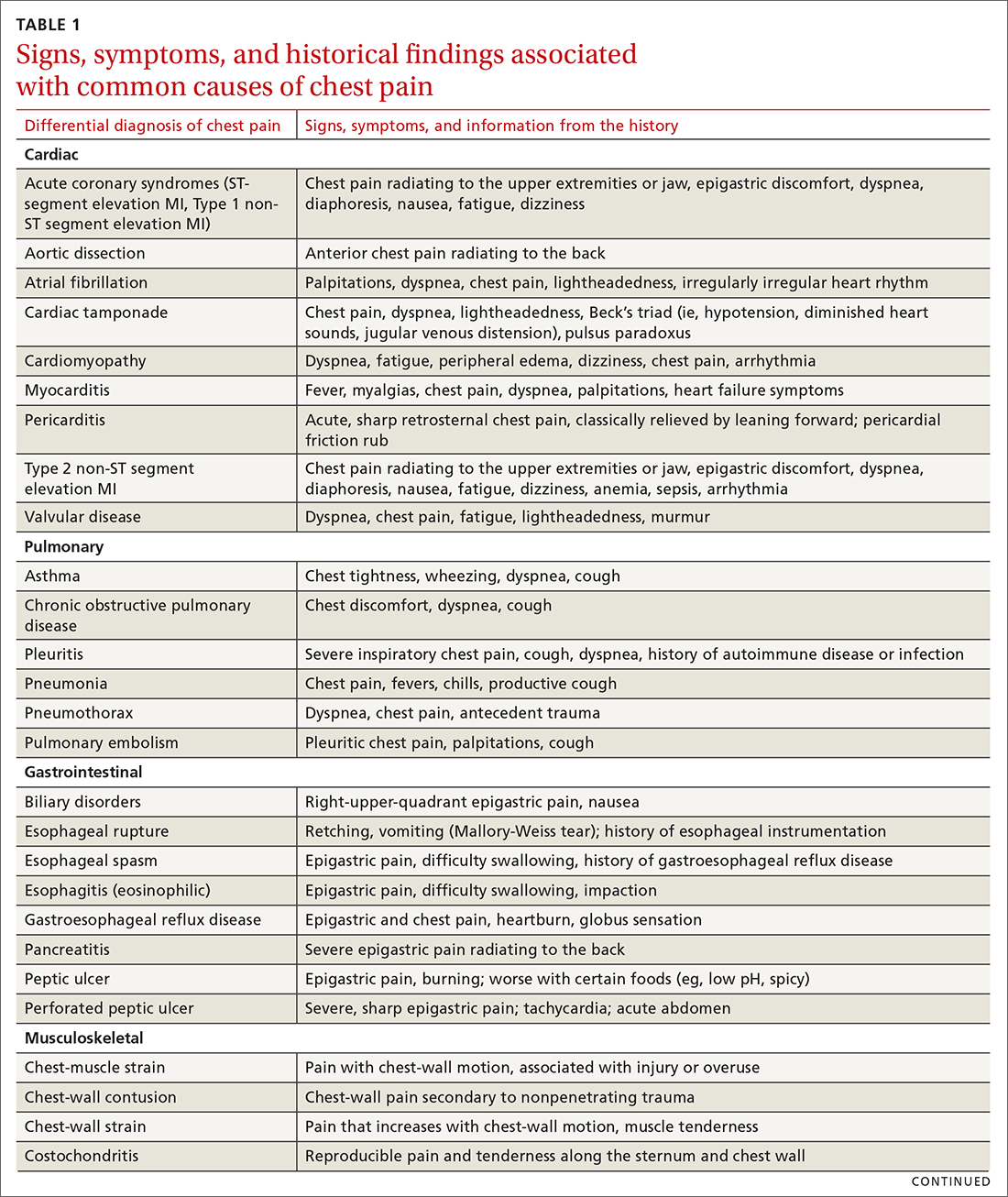
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
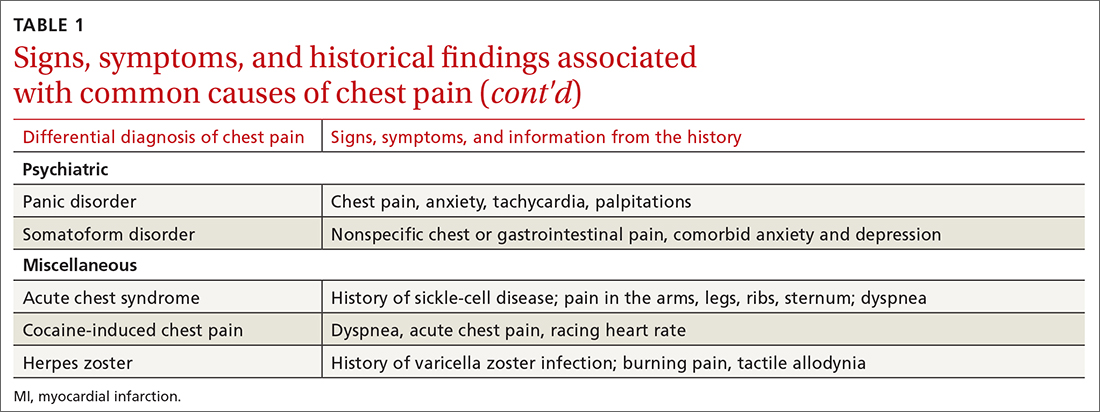
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
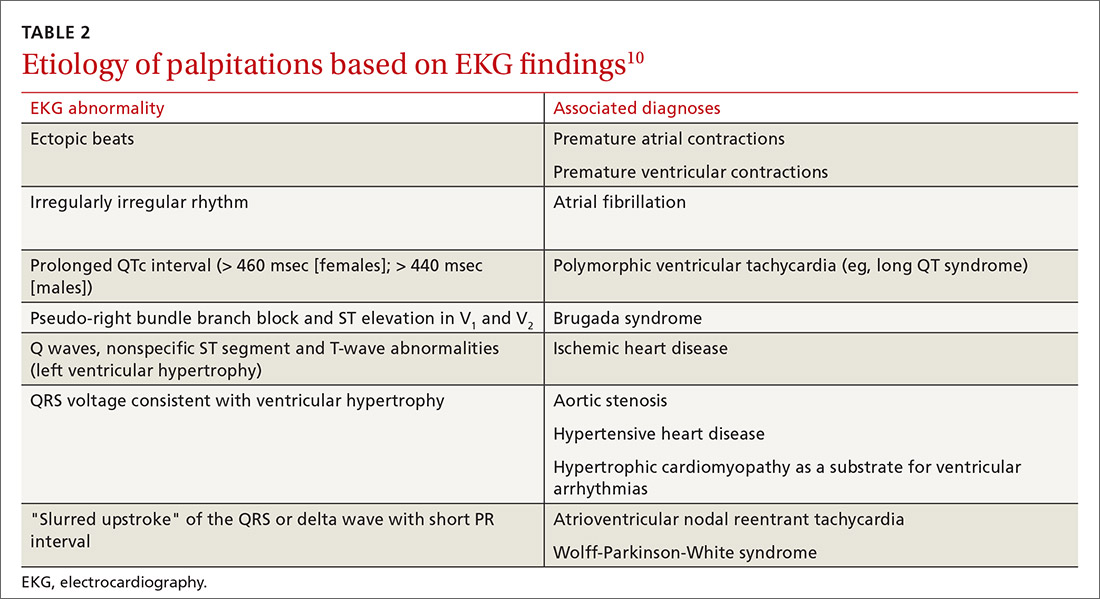
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
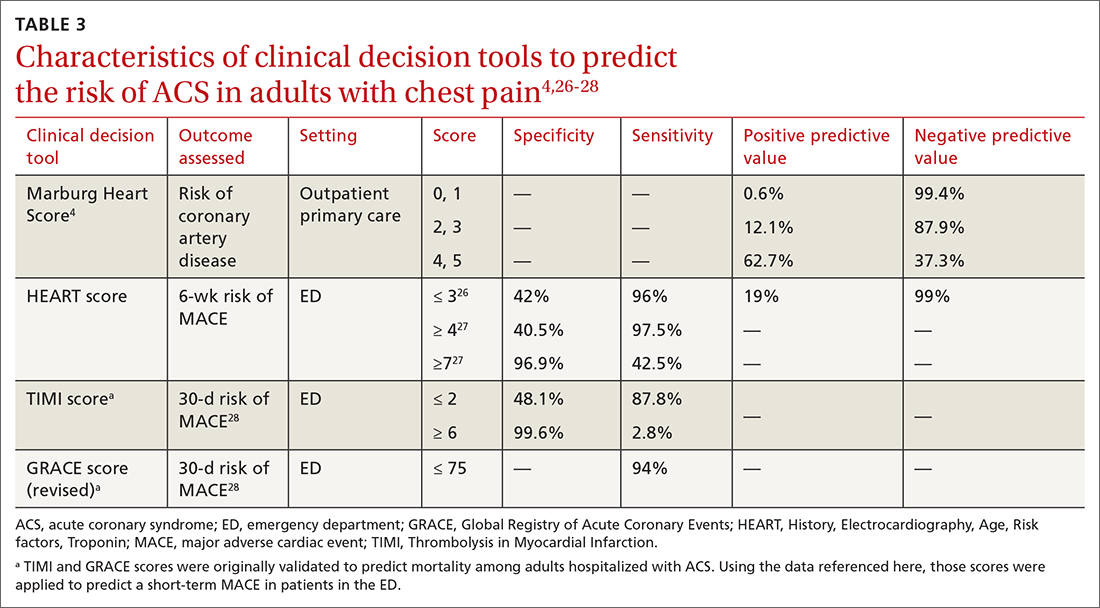
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
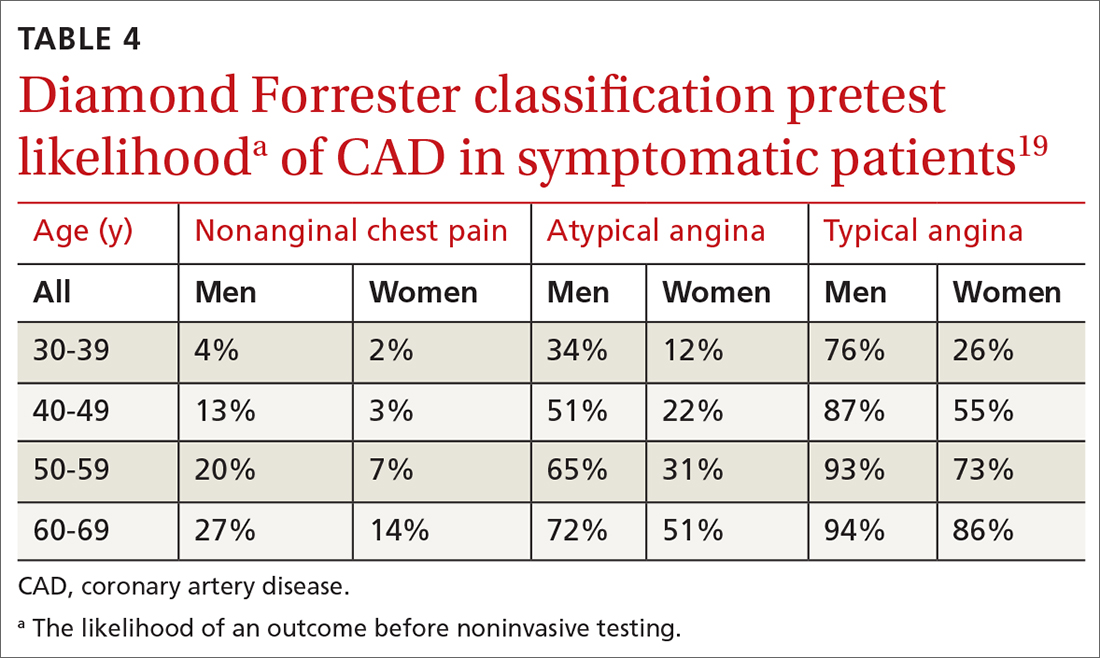
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
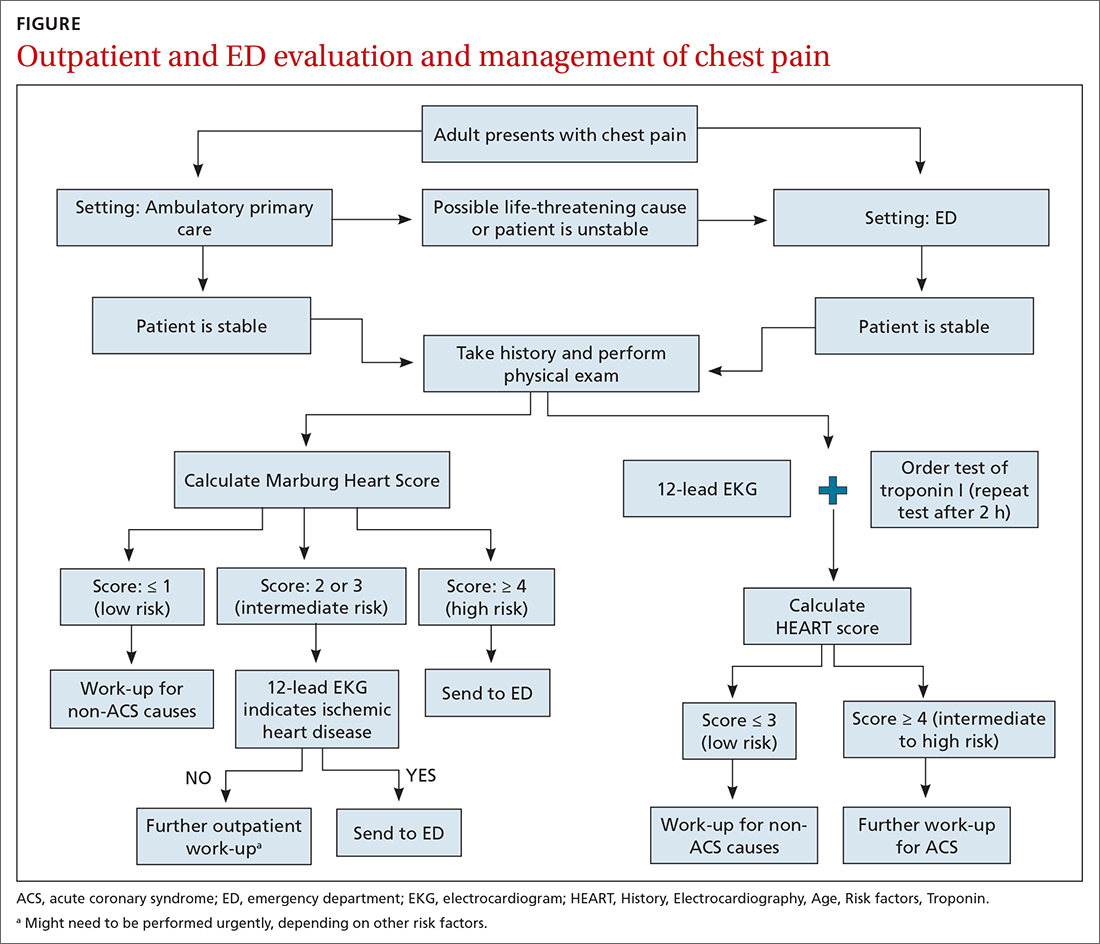
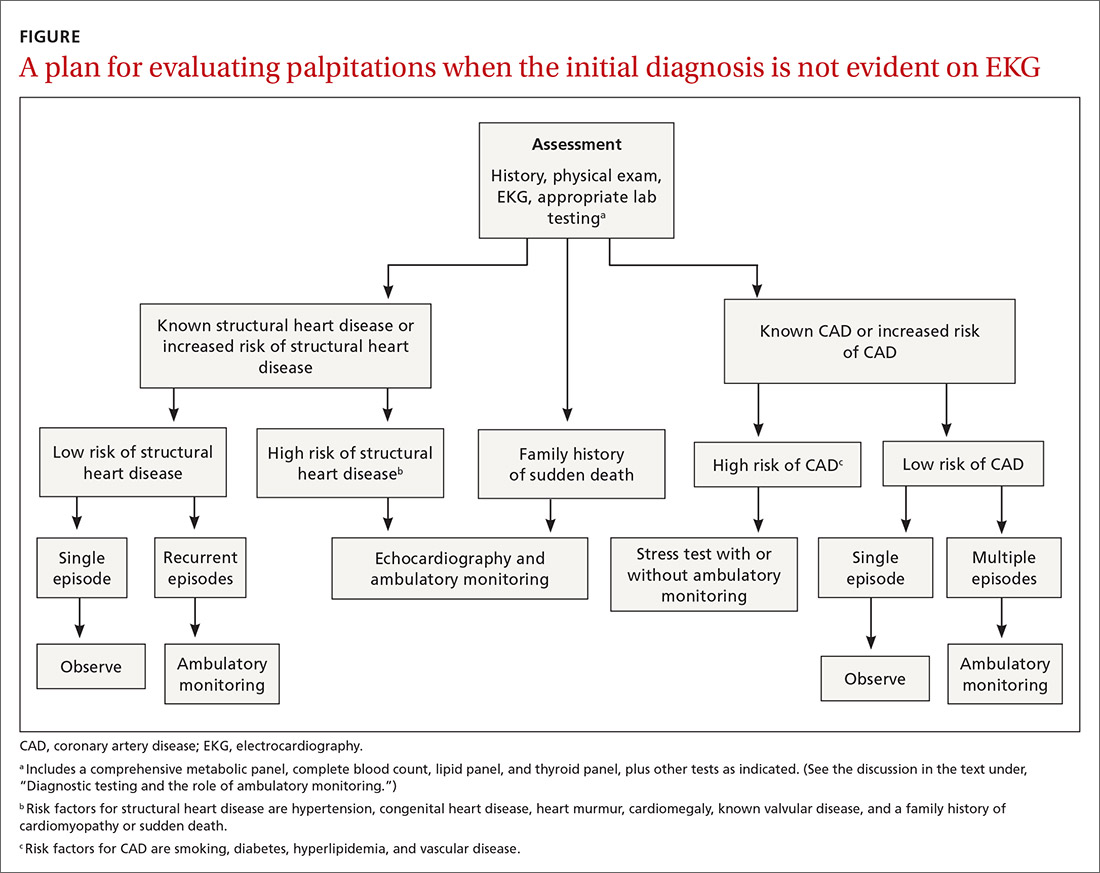
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
PRACTICE RECOMMENDATIONS
› Use the highly sensitive Marburg Heart Score to rule out coronary artery disease as a cause of chest pain in the ambulatory care setting. B
› Consider a prior normal stress test result nonpredictive of outcome in a patient presenting with chest pain. Patients with such a history of testing have a risk of a 30-day adverse cardiac event that is similar to the risk seen in patients who have never had a stress test. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
a Risk factors include hypertension, hypercholesterolemia, diabetes, obesity (body mass index > 30), smoking (current, or smoking cessation for ≤ 3 mo), and family history of CAD (ie, parent or sibling affected before 65 years of age). Atherosclerotic disease includes history of AMI, percutaneous coronary intervention or coronary artery bypass grafting, stroke, or peripheral artery disease.
Is an underlying cardiac condition causing your patient’s palpitations?
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu.
Palpitations, the sensory perception of one’s heartbeat, are reported in 16% of primary care patients, from causes that are both cardiac (ie, arrhythmias) and noncardiac.1 Palpitations are usually benign; overall mortality is approximately 1% annually. In fact, a retrospective study found no difference in mortality and morbidity between patients with palpitations and control patients without palpitations.2 However, palpitations can reflect a life-threatening cardiac condition, as we discuss in this article, making careful assessment and targeted, sometimes urgent, intervention important.3
Here, we review the clinical work-up of palpitations, recommended diagnostic testing, and the range of interventions for cardiac arrhythmias—ectopic beats, ventricular tachycardia (VT), and atrial fibrillation (AF).
Cardiac and noncardiac causes of palpitations
In a prospective cohort study of 190 consecutive patients presenting with palpitations, the cause was cardiac in 43%, psychiatric in 31%, and of a miscellaneous nature (including medication, thyrotoxicosis, caffeine, cocaine, anemia, amphetamine, and mastocytosis) in 10%; in 16%, the cause was undetermined.2 In this study, 77% of patients experienced a recurrence of palpitations after their first episode.2
Cardiac arrhythmias, a common cause of palpitations, are differentiated by site of origin—supraventricular and ventricular. Noncardiac causes of palpitations, which we do not discuss here, include metabolic and psychiatric conditions, medications, and substance use. (For a summary of the causes of palpitations, see TABLE 1.2-4)
Common complaint: ectopic beats. Premature atrial contractions (PACs; also known as premature atrial beats, atrial premature complexes, and atrial premature beats) and premature ventricular contractions (PVCs; also known as ventricular premature complexes and ventricular premature beats, and also of a variety of possible causes) result in a feeling of a skipped heartbeat or a flipping sensation in the chest.
The burden of PACs is independently associated with mortality, cardiovascular hospitalization, new-onset AF, and pacemaker implantation. In a multivariate analysis, a PAC burden > 76 beats/d was an independent predictor of mortality (hazard ratio [HR] = 1.4; 95% CI, 1.2-16); cardiovascular hospitalization (HR = 1.3; 95% CI, 1.1-1.5); new-onset AF (HR = 1.8; 95% CI, 1.4-2.2); and pacemaker implantation (HR = 2.8; 95% CI, 1.9-4.2). Frequent PACs can lead to cardiac remodeling, so more intense follow-up of patients with a high PAC burden might allow for early detection of AF or subclinical cardiac disease.5,6
A burden of PVCs > 24% is associated with an increased risk of PVC-induced cardiomyopathy and heart failure. Polymorphic PVCs are more concerning than monomorphic PVCs because the former suggests the presence of more diffuse, rather than localized, myocardial injury. The presence of frequent (> 1000 beats/d) PVCs warrants evaluation and treatment for underlying structural heart disease and ischemic heart disease. Therapy directed toward underlying heart disease can reduce the frequency of PVCs.7-9
Continue to: The diagnostic work-up
The diagnostic work-up
The most important goal of the evaluation of palpitations is to determine the presence, or risk, of structural heart or coronary artery disease (CAD) by means of the history, physical examination, and electrocardiography (EKG). Patients who have an increased risk of structural heart disease need further evaluation with echocardiography; those at increased risk of CAD should have stress testing.
Hemodynamically unstable patients need admission; patients who have a history of syncope with palpitations usually should be admitted for cardiac monitoring. Patients who have had a single episode of palpitations and have normal baseline results of laboratory testing and a normal EKG, and no risk factors for structural heart disease or known CAD, can usually be observed.3,4,10 Patients with an abnormal baseline EKG, recurrent palpitations (especially tachyarrhythmia), or significant symptoms during palpitations (syncope, presyncope, dyspnea) need further evaluation with ambulatory monitoring3,4,10 (Figure).
Take a thorough history; ask these questions
Have the patient describe the palpitations. The history should include the patient’s detailed characterization of the palpitations (sudden or gradual onset, rhythm, duration, frequency). Certain descriptions provide possible diagnostic clues:
- Palpitations lasting < 5 minutes are less likely to be of cardiac origin (likelihood ratio [LR] = 0.38; 95% CI, 0.2-0.6).4
- A patient who has a regular, rapid-pounding sensation in the neck has an increased probability of atrioventricular (AV) nodal reentrant tachycardia (AVNRT) (LR = 177; 95% CI, 25-1251); absence of this sensation decreases the likelihood of AVNRT (LR = 0.07; 95% CI, 0.03-0.2).4
- PACs and PVCs cause a sensation of a skipped heartbeat or a flipping sensation in the chest; they are not reported as a sustained rapid heartbeat.
- Patients with a supraventricular arrhythmia often report sudden onset and cessation of palpitations.
- Patients with palpitations since childhood are more likely to have supraventricular tachycardia (SVT).4
Elicit apparent precipitating and alleviating factors. The history should include notation of situations that appear to the patient to lead to palpitations (eg, context, positional variation). Palpitations that affect sleep (LR = 2.3; 95% CI, 1.3-3.9) and palpitations that occur at work (LR = 2.2; 95% CI, 1.3-5) increase the likelihood of a cardiac cause.4 Palpitations associated with sudden change in position, such as bending forward or squatting, are more likely due to AVNRT.11
Ask about aggravating factors (eg, exercise) and relieving factors (eg, rest, performing a Valsalva maneuver). Patients with SVT are often able to have palpitations terminated with a Valsalva maneuver, such as carotid sinus massage. Palpitations and syncope during exertion can be associated with hypertrophic cardiomyopathy, congenital coronary anomalies, and ion channelopathies, and can cause sudden cardiac death in athletes (estimated incidence, 1-3/100,000 person–years12).
Endeavor to identify underlying cardiac disease. A comprehensive history should also evaluate for risk factors and symptoms (chest pain, dyspnea, diaphoresis, lightheadedness, syncope) of cardiac disease, such as CAD, valvular disease, cardiomyopathy, and congenital heart disease, which increase the likelihood that the presenting complaint is a cardiac arrhythmia (LR = 2; 95% CI, 1.3-3.1).4 A history of syncope in a patient with palpitations should prompt evaluation for structural heart disease, such as aortic stenosis or hypertrophic cardiomyopathy, in which outflow-tract obstruction impairs cardiac output and, subsequently, cerebral blood flow.
Obtain additional key information. Determine the following in taking the history:
- Is there a family history of inherited cardiac disorders or sudden cardiac death?
- What prescription and over-the-counter medications is the patient taking? How does the patient characterize his or her use/intake of recreational drugs, nicotine, caffeine, and alcohol?
- Does the patient have a history of panic disorder, which lessens concern about a cardiac cause (LR = 0.2; 95% CI, 0.07-1.01)?4 (Of note: A nonpsychiatric cause can coexist in such patients, and should be considered.)
Continue to: Physical examination clues, and the utility of vagal maneuvers
Physical examination clues, and the utility of vagal maneuvers
Although most patients in whom palpitations are the presenting complaint are, in fact, asymptomatic during clinical assessment, cardiovascular examination can assist in diagnosing the arrhythmia or structural heart disease:
- Resting bradycardia increases the likelihood of a clinically significant arrhythmia (LR = 3; 95% CI, 1.27-7.0).11
- A murmur, such as a midsystolic click or holosystolic murmur, detected during the cardiac exam can indicate mitral valve prolapse; a holosystolic murmur, exacerbated upon performing a Valsalva maneuver, suggests hypertrophic cardiomyopathy.
- Visible neck pulsations detected during assessment of the jugular venous pressure, known as cannon atrial (cannon A) waves, reflect abnormal contraction of the right atrium against a closed tricuspid valve during AV dissociation. Cannon A waves have an LR of 2.68 (95% CI, 1.25-5.78) for predicting AVNRT.4
Vagal nerve stimulation. In the rare circumstance that a patient complaining of palpitations is symptomatic during assessment, several tachycardias can be detected with the use of vagal maneuvers. Interruption of the tachycardia during carotid massage suggests a tachycardia involving the AV junction (AVNRT), whereas only a temporary pause or reduction in frequency is more common in atrial flutter, AF, and atrial tachycardias. Carotid massage has no effect on the presentation of ventricular arrhythmias.10
Diagnostic testing and the role of ambulatory monitoring
Electrocardiography. All patients with palpitations should have a 12-lead EKG, which may provide diagnostic clues (TABLE 210).
Ambulatory monitoring. When the EKG is nondiagnostic, ambulatory cardiac monitoring has an established role in the diagnosis of recurrent palpitations. In a small study of patients presenting with palpitations to a general practitioner, the deduction of those practitioners was wrong more than half the time when they predicted a ≤ 20% chance of an arrhythmia based on the history, physical exam, and EKG alone13—emphasizing the importance of ambulatory monitoring in patients with recurrent palpitations.
Which monitoring system is most suitable depends on symptom frequency, availability, cost, and patient competence. Twenty-four- to 48-hour Holter monitoring can be used in cases of frequent (eg, daily) palpitations. An automatic external loop recorder can be used for less frequent (eg, every 30 days) symptoms. Most ambulatory EKG is now automatic, and therefore does not require patient activation; older manual systems require patient activation during symptoms.
Two weeks of ambulatory EKG have proved sufficient for determining that there is a cardiac basis to palpitations. The diagnostic yield of ambulatory EKG is highest during Week 1 (1.04 diagnoses per patient), compared to Week 3 (0.17 diagnoses per patient).14
Implantable loop recorders are placed subcutaneously to provide EKG monitoring for approximately 3 years. They are better suited for diagnosing infrequent palpitations. The diagnostic yield of an implantable loop recorder over the course of 1 year for the detection of an arrhythmia is 73%, compared to 21% for a 24-hour Holter monitor, electrophysiology studies, and 4 weeks of an external loop recorder.15 Implantable loop recorders are often reserved for patients with palpitations associated with unexplained recurrent syncope.15
Continue to: Lab work
Lab work. A comprehensive metabolic panel, complete blood count, lipid panel, and thyroid panel should be ordered for all patients with palpitations. Possible additional tests include a urine drug screen (when recreational drug use is suspected); cardiac enzymes; N-terminal-pro hormone B-type natriuretic peptide (when there is evidence of CAD or heart failure); and urinary catecholamines (when pheochromocytoma is suspected).
Other investigations. Echocardiography is indicated when structural heart disease is suspected (TABLE 12-4). Patients who have multiple risk factors for CAD or exertional symptoms might warrant a stress test.
Management
PACs and PVCs
Typically, patients are counseled to minimize potential adrenergic precipitants, such as smoking, alcohol, stress, and caffeine. However, limited studies have demonstrated no significant arrhythmogenic potential of a modest dose of caffeine (200 mg), even in patients with known life-threatening ventricular arrhythmias.16 Beta-blockers and nondihydropyridine calcium channel blockers (CCBs) can reduce the severity of symptoms related to premature ectopic beats and might reduce their frequency, although response is inconsistent. Use of these medications for PACs is largely based on expert opinion and extrapolated from use in other supraventricular and ventricular arrhythmias.
Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained VT due to prior myocardial infarction, left ventricular ejection fraction ≤ 40%, and inducible ventricular fibrillation or sustained VT on electrophysiological study.7
Patients with a high burden of ectopy who do not respond to treatment with AV nodal-blocking agents should be referred to Cardiology for other antiarrhythmic agents or catheter ablation. Last, asymptomatic ectopy does not need to be treated; there is no clear evidence that suppression with pharmacotherapy improves overall survival.15,17
Supraventricular tachycardia
The priority when evaluating any tachycardia is to assess the patient’s stability. Unstable patients should be treated immediately, usually with cardioversion, before an extensive diagnostic evaluation.18 Patients with wide-complex tachycardia (QRS > 120 ms) are generally more unstable and require more urgent therapy and cardiac consultation or referral. Hemodynamically stable patients with narrow-complex SVT (QRS < 120 ms) can be treated with IV adenosine, which has an 89.7% success rate.18,19 If adenosine is unsuccessful, cardioversion is indicated.
Stable patients with minimal symptoms and short episodes do not need treatment.
Continue to: Vagal maneuvers
Vagal maneuvers (eg, Valsalva maneuver; unilateral carotid massage after exclusion of a carotid bruit, with head tilted to the side opposite the massage, and not for longer than 10 seconds; or applying an ice-cold wet towel to the face) have a success rate of about 25% and are most effective when performed shortly after onset of arrhythmia. Vagal maneuvers can be used in all patients while preparing to administer medications.20
Patients who need treatment can take the “pill-in-the-pocket” approach with single-dose oral flecainide (3 mg/kg) or combined diltiazem and propranolol. Flecainide has a 94% success rate; diltiazem–propranolol has a lower success rate (61%) but a shorter time to conversion to sinus rhythm.21 Patients with sustained or recurrent episodes of SVT should be referred to a cardiologist for chronic prophylactic drug therapy or radiofrequency ablation.
Atrial fibrillation
Hemodynamically unstable patients with AF or atrial flutter, defined by the presence of angina, decompensated heart failure, hypotension, pulmonary edema, or evidence of organ hypoperfusion, should be electrically cardioverted using synchronized direct current.
Hemodynamically stable patients with a rapid ventricular rate should be treated with an IV or oral beta-blocker, CCB, or amiodarone, or electrically cardioverted. IV medications are typically preferred in the acute setting for ease and rapidity of administration; however, there is no evidence that IV formulations of beta-blockers and CCBs are superior to oral formulations. Once the ventricular rate is controlled, patients can be transitioned to an oral short-acting preparation of the selected agent, then converted to an appropriate dosage of an extended-release preparation.22
Cardioversion can be performed in patients with AF < 48 hours. In patients with AF > 48 hours, either 4 weeks of anticoagulation can be given, followed by cardioversion, or transesophageal echocardiography should be performed to evaluate for atrial thrombus; if atrial thrombus is absent, cardioversion can be performed.22 Transesophageal echocardiography might be unnecessary in patients known to have been on sustained anticoagulation.
Rate control is noninferior to rhythm control and does not decrease survival, functional capacity, or quality of life. Rate-control medications include beta-blockers, nondihydropyridine CCBs, amiodarone, and digoxin.
In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial of 4060 patients, mortality was the same with rhythm control (21.3%) and rate control (23.8%) (HR = 1.15; 95% CI, 0.99-1.34), with no difference in the incidence of cardiac death, arrhythmic death, or death due to stroke.23 In the RACE (RAte Control versus Electrical cardioversion for persistent atrial fibrillation) trial of 522 patients with persistent AF, rate control was noninferior to rhythm control (by cardioversion and drugs) for reducing morbidity and preventing cardiovascular death.24 One possible reason why the rhythm control strategy in the RACE trial did not show superiority is the low number of patients who achieved sustained sinus rhythm.25
Continue to: The recommended ventricular rate...
The recommended ventricular rate has traditionally been 60 to 80 beats/min at rest and < 110 beats/min during daily activities. However, a recent trial found fewer adverse outcomes and no change in symptoms or the outcome of hospitalization in patients randomized to more lenient control (target resting heart rate, < 110 beats/min), although the mean of the actual lenient rate achieved was 86 beats/minute.24
Rhythm control. Antiarrhythmic agents or procedural interventions can be used in patients who fail or remain symptomatic despite rate control.26 Surgical measures include AV node ablation with placement of a pacemaker; atrial pacing with an implantable atrial defibrillator; the Maze procedure (open-heart surgery) to interrupt reentrant circuits in the left atrium; and percutaneous radiofrequency or cryotherapy ablation of arrhythmogenic foci in and around the junction of the pulmonary veins and left atrium.27
There is no significant benefit to immediate catheter ablation over standard medical therapy in adults with symptomatic AF in reducing the composite outcome of death, stroke, serious bleeding, and cardiac arrest. Catheter ablation is associated with a lower AF recurrence rate (50%) than drug therapy (69%) at 3 years.28
Anticoagulation. Patients at high risk of embolic stroke based on their score on the CHA2DS2-VASca risk stratification tool (ie, a score ≥ 2) should be anticoagulated.29,30 Options include a novel oral anticoagulant (dabigatran, rivaroxaban, apixaban, or edoxaban), the preferred class of agents for nonvalvular AF, and warfarin, with a target International Normalized Ratio of 2 to 3. Novel oral anticoagulants have been compared to warfarin for prevention of stroke in AF and were found more effective than warfarin, although at the expense of an increased risk of gastrointestinal bleeding.31 Percutaneous left atrial appendage closure, using a device such as the Watchman implant, is a noninferior surgical method to prevent embolic stroke in patients who are intolerant of, or have a contraindication to, anticoagulation.32
CORRESPONDENCE
Anne Mounsey, MD, Department of Family Medicine, University of North Carolina, 590 Manning Drive, Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
2. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100:138-148.
3. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. 2018;10:387-396.
4. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302:2135-2143.
5. Lin C-Y, Lin Y-J, Chen Y-Y, et al. Prognostic significance of premature atrial complexes burden in prediction of long-term outcome. J Am Heart Assoc. 2015;4:e002192.
6. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170-178.
7. Ahn M-S. Current concepts of premature ventricular contractions. J Lifestyle Med. 2013;3:26-33.
8. Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128-134.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707-1712.
10 Raviele A, Giada F, Bergfeldt L, et al; European Heart Rhythm Association. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13:920-934.
11. Chiou C-W, Chen S-A, Kung M-H, et al. Effects of continuous enhanced vagal tone on dual atrioventricular node and accessory pathways. Circulation. 2003;107:2583-2588.
12 Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648.
13. Hoefman E, Boer KR, van Weert HCPM, et al. Predictive value of history taking and physical examination in diagnosing arrhythmias in general practice. Fam Pract. 2007;24:636-641.
14 Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128:890-895.
15. Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49:1951-1956.
16. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol. 1998;82:9I-19I.
17. Sheldon SH, Latchamsetty R, Morady F, et al. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm. 2017;14:1623-1628.
18. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506-e574.
19. Alabed S, Sabouni A, Providencia R, et al. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database Syst Rev. 2017;10:CD005154.
20. Smith GD, Fry MM, Taylor D, et al. Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev. 2015;2015:CD009502.
21. Alboni P, Tomasi C, Menozzi C, et al. Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548-553.
22. King DE, Dickerson LM, Sack JL. Acute management of atrial fibrillation: Part I. Rate and rhythm control. Am Fam Physician. 2002;66:249-256.
23. Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
24. Van Gelder IC, Groenveld HF, Crijns HJGM, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
25. Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
26. Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049.
27. Ramlawi B, Bedeir K. Surgical options in atrial fibrillation. J Thorac Dis. 2015;7:204-213.
28. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274.
29. Dooley P, Doolittle J, Knauss K, et al. Atrial fibrillation: effective strategies using the latest tools. J Fam Pract. 2017;66:16-26.
30. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):CD006186.
31. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
32. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998.
PRACTICE RECOMMENDATIONS
› Order echocardiography for patients who have palpitations and risk factors for structural heart disease. C
› Order stress testing for patients who have exertional symptoms or multiple risk factors for coronary artery disease. C
› Evaluate all patients who have syncope associated with their palpitations for a cardiac cause. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Head & neck cancers: What you’ll see, how to proceed
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
PRACTICE RECOMMENDATIONS
› Do not treat a neck mass with antibiotics unless it has features consistent with infection. C
› Order laryngoscopy for all patients with hoarseness that does not resolve after 3 months—or sooner, if malignancy is suspected. C
› Order ultrasonography-guided fine-needle aspiration for diagnostic evaluation of salivary gland masses. B
› Manage a thyroid nodule based on its sonographic features, including size, consistency, and the presence of concerning features. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Best timing for measuring orthostatic vital signs?
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
PRACTICE CHANGER
Measure orthostatic vital signs within 1 minute of standing to most accurately correlate dizziness with long-term adverse outcomes. 1
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, prospective cohort study with patient-oriented outcomes and good follow-up.
Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316-1323.
Time to conception after miscarriage: How long to wait?
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
EVIDENCE-BASED ANSWER:
An interpregnancy interval (IPI) of < 6 months following miscarriage is associated with an increased live birth rate in subsequent pregnancy, lower risks of preterm birth and subsequent miscarriage, and no difference in rates of stillbirth, pre-eclampsia, and low birth weight infants (strength of recommendation [SOR]: A, well-done meta-analysis). (IPI is defined as the time between the end of one pregnancy and the last menstrual period of a subsequent one.)
A very short IPI (< 3 months), when compared with an IPI of 6 to 18 months, is associated with the lowest rate of subsequent miscarriage (SOR: B, cohort study). However, for women who experience a pregnancy loss at 14 to 19 weeks’ gestation, an IPI < 3 months is associated with an increased risk of miscarriage or birth before 24 weeks’ gestation (SOR: B, cohort study).
Women with a short IPI following miscarriage may be at increased risk for anxiety and depression in the first trimester of the subsequent pregnancy (SOR: B, cohort study).
How Do These 3 Diabetes Agents Compare in Reducing Mortality?
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
How do these 3 diabetes agents compare in reducing mortality?
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
PRACTICE CHANGER
Consider adding a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a glucagon-like peptide 1 (GLP-1) agonist to the treatment regimen of patients with poorly controlled type 2 diabetes—especially those with higher CV risk. Doing so can reduce all-cause and cardiovascular (CV) mortality 1
STRENGTH OF RECOMMENDATION
B: Based on a network meta-analysis of 236 randomized controlled trials.
Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
Treating Migraines: It’s Different for Kids
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
Does niacin decrease cardiovascular morbidity and mortality in CVD patients?
EVIDENCE SUMMARY
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5
Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
EVIDENCE SUMMARY
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5
Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
EVIDENCE SUMMARY
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5
Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE BASED ANSWER:
No. Niacin doesn’t reduce cardiovascular disease (CVD) morbidity or mortality in patients with established disease (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs] and subsequent large RCTs).
Niacin may be considered as monotherapy for patients intolerant of statins (SOR: B, one well-done RCT).