User login
Treating migraines: It’s different for kids
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Do not prescribe amitriptyline or topiramate as preventive therapy for migraine in children; both drugs are no better than placebo for this population and are associated with increased rates of adverse events.1,2
STRENGTH OF RECOMMENDATION
A: Based on a single double-blind randomized control trial (RCT) and supported by a meta-analysis of 4 RCTs.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
Does Fish Oil During Pregnancy Help Prevent Asthma in Kids?
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
Does fish oil during pregnancy help prevent asthma in kids?
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Fish oil supplementation taken by women in the third trimester of pregnancy can reduce the risk of persistent wheeze, asthma, and infections of the lower respiratory tract in their children.1
STRENGTH OF RECOMMENDATION
B: Based on 2 double-blinded randomized controlled trials (RCTs).
Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.1
Prescribe This Combined OC With CV Safety in Mind
A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).
A 28-year-old woman presents to your office for a routine health maintenance exam. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a pa
In general, when compared with nonusers, women who use COCs have a two- to four-fold increase in risk for venous thromboembolism (VTE) and an increased risk for myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk for VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk for VTE than other preparations, despite similar estrogen content.7 The FDA produced a similar statement that same year, recommending that providers carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks for ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One COC comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks for pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included nearly 5 million women ages 15 to 49, living in France, who had at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks for first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 µg) or high-dose estrogen (30-40 µg) combined with one of five progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI, with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 for PE, 0.82 for ischemic stroke, and 0.56 for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7,143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs for PE but not arterial events (2.16 for desogestrel and 1.63 for gestodene). For PE, the ARR with levonorgestrel compared to desogestrel and gestodene, respectively, was 19/100,000 and 12/100,000 person-years of use (NNH, 5,263 and 8,333, respectively). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk for PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks for PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen + levonorgestrel confer lowest risk
Prior studies have shown that COCs increase the risk for PE and may also increase the risks for ischemic stroke and MI.3,11 Studies have also suggested that a higher dose of estrogen in COCs is associated with an increased risk for VTE.11,12 This study shows that 20 µg of estrogen combined with levonorgestrel is associated with the lowest risks for PE, MI, and ischemic stroke.
CAVEATS
Cohort study, no start date, incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk for VTE is highest in the first three months to one year of COC use.12 Data on tobacco use, a significant independent risk factor for arterial but not venous thromboembolism, was incomplete; however, in other studies, it has only marginally affected outcomes.3,13
CHALLENGES TO IMPLEMENTATION
Increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 µg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[7]:454-456).
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. FDA. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed July 5, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP monthly report on safety concerns, guidelines and general matters. 2012. www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed July 5, 2017.
8. FDA. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed July 5, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
Which combined OC to prescribe with CV safety in mind?
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
When prescribing combined oral contraceptives, choose one containing levonorgestrel and low-dose estrogen (20 mcg) to minimize the risks of pulmonary embolism, ischemic stroke, and myocardial infarction.
STRENGTH OF RECOMMENDATION
B: Based on a good quality, patient-oriented cohort study.
Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.1
“Cold Turkey” Works Best for Smoking Cessation
A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).
A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
“Cold turkey” works best for smoking cessation
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 43-year-old man has a 35-pack-year smoking history and currently smokes a pack of cigarettes a day. He is eager to quit smoking after recently learning that a close friend of his has been diagnosed with lung cancer. He asks you whether he should quit “cold turkey” or gradually. What would you recommend?
Between 2013 and 2014, one in 5 American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions both alone and in combination increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual vs abrupt smoking cessation methods.5
A previous Cochrane review of 10 randomized controlled trials demonstrated no significant difference in quit rates between gradual cigarette reduction leading up to a designated quit day and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of 62,508 smokers who presented to nationwide cessation services. The researchers found that older participants (≥45 years of age) and heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
Quitting “cold turkey” is better than gradual cessation at 6 months
Lindson-Hawley, et al, conducted a randomized, controlled, non-inferiority trial in England to assess if gradual cessation is as successful as abrupt cessation as a means of quitting smoking.1 The primary outcome was abstinence from smoking at 4 weeks, assessed using the Russell Standard, a set of 6 standard criteria (including validation by exhaled carbon monoxide concentrations of <10 ppm) used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Study participants were recruited via letters from their primary care practice inviting them to call the researchers if they were interested in participating in a smoking cessation study. Almost 1100 people inquired about the study. In the end, 697 were randomized to either the abrupt-cessation group (n=355) or the gradual-cessation group (n=342). Baseline characteristics between the 2 groups were similar.
All participants were asked to schedule a quit date for 2 weeks after their enrollment. Patients randomized to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT) (gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the 2 weeks leading up to the quit date, along with instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomized to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those 2 weeks they were given nicotine patches (because the other group received them and some evidence suggests that precessation NRT increases quit rates), but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, 21 mg/d nicotine patches, and the participant’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice weekly for 2 weeks before the quit date, the day before the quit date, weekly for 4 weeks after the quit date, and 8 weeks after the quit date.
The chosen non-inferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for non-inferiority; in fact, 4-week abstinence was significantly more likely in the abrupt-cessation group (49%) than in the gradual-cessation group (39.2%) (RR=0.80; 95% confidence interval [CI], 0.66-0.93; number needed to treat [NNT]=10). Similarly, secondary outcomes of 8-week and 6-month abstinence rates showed superiority of abrupt over gradual cessation. At 6 months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR=0.71; 95% CI, 0.46-0.91; NNT=15).
Patients’ preferred method of cessation plays a role
The investigators also found a difference in successful cessation based on the participants preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at 4 weeks than participants who preferred gradual cessation (52.2% vs 38.3%; P=.007).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation: 4-week abstinence was seen in 34.6% of patients who preferred gradual cessation and were allocated to gradual cessation and in 42% of patients who preferred gradual cessation but were allocated to abrupt cessation (P=.152).
WHAT'S NEW
Higher quality than previous studies and added element of preference
This large, well-designed, non-inferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were ultimately randomized.
CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at 4 weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of participants included in the study, along with the relatively small number of patients lost to follow-up, minimizes this weakness.
The participants were largely white, which may limit generalizability to non-white populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥21 cigarettes/d) were more likely to quit gradually than abruptly, so results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Finding the time and staff for considerable behavioral support
One important challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse 7 times in the first 6 weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center or Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL, for the US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Preventive Medicine. 2014;63:96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Counsel patients who want to quit smoking that abrupt smoking cessation is more effective for long-term abstinence than taking a gradual approach.
STRENGTH OF RECOMMENDATION
B: Based on one well-designed, randomized controlled trial.
Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.1
Need an Add-on to Metformin? Consider This
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
A 58-year-old woman with T2DM and heart failure returns to your office for follow-up. She has been on the maximum dose of metformin alone for the past six months, but her A1C is now 7.8%. She wants to avoid injections. What do you recommend?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attaining glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular (CV) risk, so the choice of a second drug is an important one.2 While the proven mortality benefit, wide availability, and low cost of metformin make it well-established as initial pharmacotherapy, no second-choice drug has amassed enough evidence of benefit to become the add-on therapy of choice.
The professional societies are of little assistance; dual-therapy recommendations from the American Diabetes Association and the European Association for the Study of Diabetes do not specify a preference.3 Although the American Association of Clinical Endocrinologists/American College of Endocrinology suggest a hierarchy of choices, it is based on expert consensus recommendations.4
A look at the options
Options for add-on therapy include sulfonylureas, thiazolidines, DPP-4 inhibitors, sodium glucose cotransporter 2 inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers frequently prescribe sulfonylureas after metformin because they are low in cost, have long-term safety data, and are effective at lowering A1C. They work by directly stimulating insulin secretion via pancreatic ß-cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, sulfonylureas carry significant risk for hypoglycemia (relative risk [RR], 4.57) and weight gain (average, 2.06 kg), compared to placebo.5
DPP-4 inhibitors, on the other hand, induce insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer CV events and less hypoglycemia than sulfonylureas but were subsequently linked to an increased risk for heart failure–related hospitalization.7
A recent study provides more data on the effects of DPP-4s added to metformin.1
STUDY SUMMARY
DPP-4s as effective, less risky
This observational cohort study compared DPP-4 inhibitors and sulfonylureas when combined with metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse CV events (defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. The study included data from the National Health Insurance Research Database in Taiwan on more than 70,000 patients (ages 20 and older) with diagnosed T2DM. Individuals adherent to metformin were considered to be enrolled in the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Participants were then matched by propensity score into 10,089 pairs, each consisting of one DPP-4 inhibitor user and one sulfonylurea user.
After mean follow-up of 2.8 years, the investigators used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis stratified by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Similar results were also obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—compared with those who used sulfonylureas—had a lower risk for all-cause mortality (366 vs 488 deaths; hazard ratio [HR], 0.63; number needed to treat [NNT], 117), major cardiac events (209 vs 282 events; HR, 0.68; NNT, 191), ischemic stroke (144 vs 203 strokes; HR, 0.64; NNT, 246), and hypoglycemia (89 vs 170 events; HR, 0.43; NNT, 201). There were no significant differences in the occurrence of MIs (69 vs 88 MIs; HR, 0.75) or the number of hospitalizations for heart failure (100 vs 100 events; HR, 0.78) between the two groups.
WHAT’S NEW
Lower risks for death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, CV events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk for heart failure hospitalization. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments (including DPP-4 inhibitors and GLP-1 agonists) found no increased risk for heart failure hospitalization with DPP-4 inhibitors, compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this particular study, there may have been unmeasured but significant patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when added to metformin.6
Another caveat is that the results from this study group may not be generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk for T2DM at a lower BMI than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas (eg, glyburide) carry a higher risk for hypoglycemia, which could bias the results.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for secondline pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin; it may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s are more expensive
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. For patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(1):42-44.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1).
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22: 84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. www.goodrx.com/gliptins. Accessed January 4, 2017.
Can mobile technology improve weight loss in overweight and obese patients?
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).
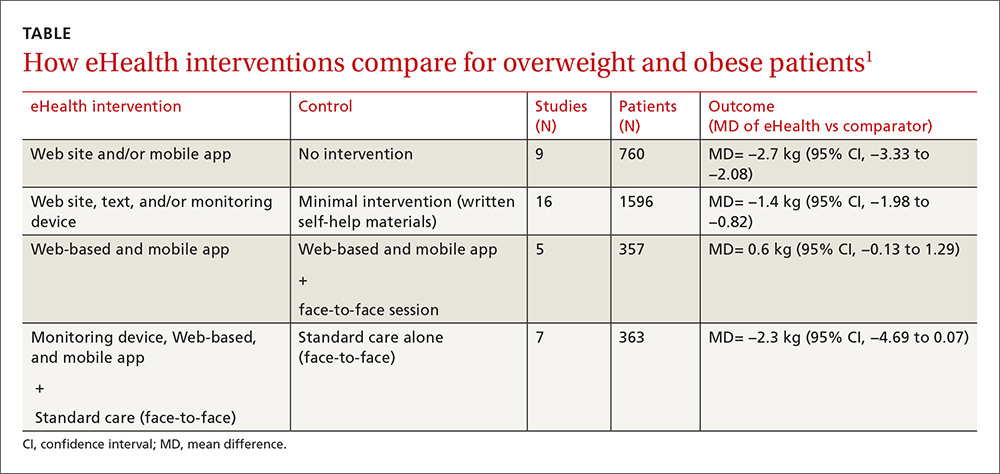
Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
EVIDENCE SUMMARY
A systematic review and meta-analysis of 84 moderate- to high-quality RCTs with 24,010 patients evaluated the use of “eHealth” interventions in preventing and treating overweight and obesity in adults 35 to 65 years of age (75% female).1 The studies included 183 active intervention arms with durations as long as 24 months (64% <6 months, 46% >6 months). The term eHealth included all forms of information technology used to deliver health care, but predominantly the Internet (Web site/Web-based), e-mail, and text messaging. Sixty percent (84) of eHealth interventional arms used one modality and 34% (47) used 2. Some intervention arms included non-eHealth modalities, such as paper-based measures and counseling.
The eHealth interventions were associated with significantly greater weight loss than minimal or no intervention (TABLE).1 Comparing eHealth interventions with no intervention showed significant differences by eHealth type (P=.05). The greatest weight loss accompanied interventions that combined Web-based measures with a non-eHealth intervention, (mean difference [MD]= −3.7 kg; 95% confidence interval [CI], −4.46 to −2.94), followed by mobile interventions alone (MD= −2.4 kg; 95% CI, −4.09 to −0.71) and Web-based interventions alone (MD= −2.2 kg; 95% CI, −2.98 to −1.44).

Similarly, comparing combined interventions (eHealth + eHealth or eHealth + non-eHealth) with a minimal intervention control showed a trend for difference by eHealth type (P=.005). Only a combination of eHealth with non-eHealth interventions resulted in significantly greater weight loss (Web site + non-eHealth: MD= −2.7 kg; 95% CI, −3.76 to −1.54; text + non-eHealth: MD= −1.8 kg; 95% CI, −2.49 to −1.12; computer + non-eHealth: MD=1.1 kg; 95% CI, −1.36 to −0.89).
Personal coaching plus smartphone monitoring beats interactive app
A 3-arm RCT of 385 overweight and obese participants (mean body mass index [BMI], 35 kg/m2) 18 to 35 years of age compared the effectiveness of weight loss interventions delivered by interactive smartphone application (CP [cell phone]), personal coaching enhanced by smartphone self-monitoring (PC), and usual care (control).2 The PC arm attended 6 weekly group sessions and received monthly phone calls. The usual care arm received 3 handouts on healthy eating and physical activity.
The CP arm showed the least amount of weight loss (−0.9 kg, −1.5 kg, and −1.0 kg at 6, 12, and 24 months, respectively) and no significant difference compared with controls at all measurement points. The PC arm had significantly greater weight loss than controls at 6 months (−1.9 kg; 95% CI, −3.17 to −0.67) and significantly greater weight loss than CP at 6 months (−2.2 kg; 95% CI, −3.42 to −0.97) and 12 months (−2.1 kg; 95% CI, −3.94 to −0.27). After 24 months, however, there was no significant difference in mean weight loss among treatment arms.
Automated behavioral program reduced weight and waist circumference
An RCT of 339 prediabetic, overweight, and obese patients 30 to 69 years old (mean BMI, 31 kg/m2) compared the effectiveness of Alive-PD, a fully automated, tailored, behavioral program, to usual care (control) for diabetes prevention.3 In addition to behavioral support, the program included weekly emails, Web-based tracking, a mobile phone app, and automated phone calls.
At 6 months, the intervention group had significantly greater mean weight loss (−3.4 kg vs −1.3 kg; P<.001), mean BMI (−1.1 kg/m2 vs −0.4 kg/m2; P<
Web-based program improves weight loss at 3 months, but not 12 months
An RCT of 65 overweight and obese participants (mean BMI, 32 kg/m2) with at least one cardiovascular risk factor compared the effect of a Web-based program with usual care on weight change at 3, 6, and 12 months.4 Participants in the intervention group were provided with Bluetooth-enabled scales and accelerometer activity bands to allow daily uploads. The Web-based program also provided weekly feedback based on the participant’s performance and a food diary.
The Web-based group had significantly greater weight loss at 3 months (mean= −3.4 kg [95% CI, −4.70 to −2.13] vs −0.5 kg [95% CI, −1.55 to 0.52]; P<.001) and 6 months (mean= −3.4 kg [95% CI, −4.95 to −1.98] vs −0.8 kg [95% CI, −2.23 to 0.61]; P=.02). At 12 months, however, the groups showed no significant difference (mean= −2.4 kg [95% CI, −3.48 to −0.97] vs −1.8 kg [95% CI, −3.15 to −0.44]; P=.77).
RECOMMENDATIONS
Guidelines from the American College of Cardiology, American Heart Association, and Obesity Society state that electronically delivered weight-loss programs may be prescribed, but may result in smaller weight loss than face-to-face interventions (SOR: B, moderate evidence from RCTs with some limitations or non-randomized trials).5
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
1. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16:376-392.
2. Svetkey LP, Batch BC, Lin P, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23:2133-2141.
3. Block G, Azar K, Romanelli R, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240.
4. Watson S, Woodside J, Ware L, et al. Effect of a web-based behavior change program on weight loss and cardiovascular risk factors in overweight and obese adults at high risk of developing cardiovascular disease: randomized controlled trial. J Med Internet Res. 2015;17:e177.
5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes, this technology can help in the short term. Mobile technology compared with minimal or no intervention increases short-term (<6 months) weight loss (1.4 to 2.7 kg) in overweight and obese patients (strength of recommendation [SOR]: A, meta-analysis of good-quality studies and randomized controlled trials [RCTs]).
Interventions that combine nonelectronic measures with mobile technology increase weight loss more effectively (3.7 kg) than no intervention (SOR: A, meta-analysis of good-quality studies and RCTs).
Using mobile technology shows no significant benefits for weight loss after 12 months (SOR: A, multiple good-quality RCTs).
Need an add-on to metformin? Consider this
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Consider a dipeptidyl peptidase-4 inhibitor before a sulfonylurea for patients with type 2 diabetes mellitus who require therapy in addition to metformin.
Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.1
STRENGTH OF RECOMMENDATION
B: Based on limited-quality, patient-oriented data from a high-quality, population-based cohort study.