User login
Transition Readiness Assessment for Sickle Cell Patients: A Quality Improvement Project
From the St. Jude Children’s Research Hospital, Memphis, TN.
This article is the fourth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the use of quality improvement (QI) methodology to implement an assessment tool to evaluate transition readiness in youth with sickle cell disease (SCD).
- Methods: Plan-Do-Study-Act (PDSA) cycles were run to evaluate the feasibility and effectiveness of a provider-based transition readiness assessment.
- Results: Seventy-two adolescents aged 17 years (53% male) were assessed for transition readiness from August 2011 to June 2013. Results indicated that it is feasible for a provider transition readiness assessment (PTRA) tool to be integrated into a transition program. The newly created PTRA tool can inform the level of preparedness of adolescents with SCD during planning for adult transition.
- Conclusion: The PTRA tool may be helpful for planning and preparation of youth with SCD to successfully transition to adult care.
Sickle cell disease (SCD) is one of the most common genetic disorders in the world and is caused by a mutation producing the abnormal sickle hemoglobin. Patients with SCD are living longer and transitioning from pediatric to adult providers. However, the transition years are associated with high mortality [1–4], risk for increased utilization of emergency care, and underutilization of care maintenance visits [5,6]. Successful transition from pediatric care to adult care is critical in ensuring care continuity and optimal health [7]. Barriers to successful transition include lack of preparation for transition [8,9]. To address this limitation, transition programs have been created to help foster transition preparation and readiness.
Often, chronological age determines when SCD programs transfer patients to adult care; however, age is an inadequate measure of readiness. To determine the appropriate time for transition and to individualize the subsequent preparation and planning prior to transfer, an assessment of transition readiness is needed. A number of checklists exist in the unpublished literature (eg, on institution and program websites), and a few empirically tested transition readiness measures have been developed through literature review, semi-structured interviews, and pilot testing in patient samples [10–13]. The Transition Readiness Assessment Questionnaire (TRAQ) and TRxANSITION scale are non-disease-specific measures that assess self-management and advocacy skills of youth with special health care needs; the TRAQ is self-report whereas the TRxANSITION scale is provider-administered [10,11]. Disease-specific measures have been developed for pediatric kidney transplant recipients [12] and adolescents with cystic fibrosis [13]. Studies using these measures suggest that transition readiness is associated with age, gender, disease type, increased adolescent responsibility/decreased parental involvement, and adherence [10–12].
For patients with SCD, there is no well-validated measure available to assess transition readiness [14]. Telfair and colleagues developed a sickle cell transfer questionnaire that focused on transition concerns and feelings and suggestions for transition intervention programming from the perspective of adolescents, their primary caregivers, and adults with SCD [15]. In addition, McPherson and colleagues examined SCD transition readiness in 4 areas: prior thought about transition, knowledge about steps to transition, interest in learning more about the transition process, and perceived importance of continuing care with a hematologist as an adult provider [8]. They found that adolescents in general were not prepared for transition but that readiness improved with age [8]. Overall, most readiness measures have involved patient self-report or parent proxy report. No current readiness assessment scales incorporate the provider’s assessment, which could help better define the most appropriate next steps in education and preparation for the upcoming transfer to adult care.
The St. Jude Children’s Research Hospital SCD Transition to Adult Care program was started in 2007 and is a companion program to the SCD teen clinic, serving 250 adolescents aged 12 to 18 years. The transition program curriculum addresses all aspects of the transition process. Based on the curriculum components, St. Jude developed and implemented a transition readiness assessment tool to be completed by providers in the SCD transition program. In this article, we describe our use of quality improvement (QI) methodology to evaluate the utility and impact of the newly created SCD transition readiness assessment tool.
Methods
Transition Program
The transition program is directed by a multidisciplinary team; disciplines represented on the team are medical (hematologist, genetic educator, physician assistant, and nurse coordinators), psychosocial (social workers), emotional/cognitive (psychologists), and academic (academic coordinator). In the program, adolescents with SCD and their families are introduced to the concept of transition to adult care at the age of 12. Every 6 months from 12 to 18 years of age, members of the team address relevant topics with patients to increase patients’ disease knowledge and improve their disease self-management skills. Some of the program components include training in completing a personal health record (PHR), genetic education, academic planning, and independent living skills.
Needs Assessment
Prior to initiation of the project, members of the transition program met monthly to informally discuss the progress of patients who were approaching the age of transition to adult care. We found that adolescents did not appear to be ready or well prepared for transition, including not being aware of the various familial and psychosocial issues that needed to be addressed prior to the transfer to adult care. We realized that these discussions needed to occur earlier to allow more time for preparation and transition planning of the patient, family, and medical team. In addition, members of the team each has differing perspectives and did not have the same information with regard to existing familial and psychosocial issues. The discussions were necessary to ensure all team members had pertinent information to make informed decisions about the patient’s level of transition readiness. Finally, our criteria for readiness were not standardized or quantifiable. As a result, each patient discussion was lengthy, not structured, and not very informative. In 2011, a core group from the transition team attended a Health Resources Services Administration–sponsored Hemoglobinopathies Quality Improvement Workshop to receive training in QI processes. We decided to create a formal, quantitative, individualized assessment of patients’ progress toward transition at age 17.
Readiness Assessment Tool
The emotional/cognitive domain checklist was developed by the pediatric psychologist and pediatric neuropsychologist. Because the psychology service is set up to see patients referred by the medical team and is unable to see all patients coming to hematology clinic, the emotional/cognitive checklist is based on identifying previous utilization of psychological services including psychotherapy and cognitive testing and determining whether initiation of services is warranted. The academic domain checklist was developed by the academic coordinator who serves as a liaison between the medical team and the school system. This checklist assesses whether the adolescent is meeting high school graduation requirements, able to verbalize an educational/job training plan, on track with future planning (eg, completed required testing), knowledgeable about community educational services, and able to self-advocate (eg, apply for SSI benefits).
Items within each domain have equal value (ie, each question on the checklist is worth 1 point) and the sum of points yields the quantifiable assessment of how well patients are performing in each area of their health. Assessment meetings occur monthly when eligible patients are discussed. Domains are evaluated by the health care provider responsible for his/her own domain (eg, social worker completes the psychosocial domain, the academic coordinator completes the academic domain, etc.).
PDSA Methodology
Cycle 1
The objective of the first cycle was to assess feasibility and acceptability of the assessment tool. Patients were assessed during the month of their 17th birthday. Fourteen out of 16 eligible patients (87.5%) were assessed: 1 patient was lost to follow-up, and 1 patient inadvertently was not included in the assessment due to an administrative error. Feedback from the first cycle revealed that some items on the emotional/cognitive domain checklist were not clearly defined, and there was some overlap with the psychosocial domain checklist. Additionally, some items were not readily assessed by psychology based on the structure of psychology services at the institution. Not all patients are seen by psychology; patients are referred to psychology by the team and appointments occur in the psychology clinic and were not well-integrated within the hematology clinic visit.
Cycle 2
The second cycle addressed some of the problems identified during Cycle 1. The emotional/cognitive domain checklist was revised to reflect psychology clinic utilization (psychotherapy and testing) and a section was added where team members could indicate individualized action plans. Seventeen patients out of 18 eligible patients were assessed (94.4%): 1 patient was lost to follow-up. At the conclusion of this cycle, we found that several patients had not completed certain transition program components, such as genetic education or their PHR. Therefore, we decided that we needed to indicate this and create a Plan of Action (POA) to ensure completion of program components. The POA indicated which components were outstanding, when these components would be completed, and when the team would discuss the patient again to track their progress with program components (eg, 6 months later).
Cycle 3
Following a few months using the assessment process, each member of the team provided feedback about their observations from the second cycle. The third cycle of the PDSA addressed some of the barriers identified in Cycle 2 by adding the POA and timeline for reassessment. With this information, the nurse case manager was able to identify and contact families who had significant gaps in the learning curriculum. Additionally, services such as psychological testing were scheduled in a timely manner to address academic problems and to provide rationale for accommodations and academic/vocational services before patients transferred care to the adult provider. With the number of assessed patients increasing, it was determined that a reliable tracking system to monitor progress was essential. Thus, a transition database was created to document the domain scores, individualized plan of action, and other components of the transition program, such as medical literacy quiz scores, completion of pre-transfer visits to adult providers, and completion of the PHR. During this cycle, 20 patients were assessed out of a total of 22 eligible patients (90.9%); 2 patients were lost to follow-up.
Cycle 4
This cycle is currently underway and comprises monthly assessments of eligible 17-year-old patients with SCD. From January 2013 to May 2013 we have assessed 100% of the eligible patients (21/21). All information obtained through the assessment tool is added to the transition database. Future adjustments and modifications are planned for this tool as we continue to evaluate its impact and value.
Discussion
The transition readiness assessment tool was developed to evaluate adolescent patients with SCD aged 17 years regarding their progress in the transition program and level of transition readiness. Most transition readiness measures available in the literature consider the patient and parent perspective but do not include the health care provider perspective or determine if the patient received the information necessary for successful transition. Our readiness assessment tool has been helpful in providing a structured and quantifiable means to identify at-risk patients and families prior to the transfer of care and revealing important gaps in transition planning. It also provides information in a timely manner about points of intervention to ensure patients receive adequate preparation and services (eg, psychological/neuropsychological testing). Additionally, monthly meetings are held during which the tool is scored and discussed, providing an opportunity for members of the transition team to examine patients’ progress toward transition readiness. Finally, completing an individualized tool in a multidisciplinary setting has the added benefit of encouraging increased staff collaboration and creating a venue for ongoing re-evaluation of the QI process.
We achieved our objective of completing the assessment tool for 80% of eligible patients throughout the cycles. The majority of our nonassessed patients was lost to follow-up and had not had a clinic visit in 2 to 3 years. Implementing the tool has provided us with an additional mechanism to verify transition eligibility and has afforded the transition program a systematic way to screen and track patients who are approaching the age of transition and who may have not been seen for an extended period of time. As with any large program following children with special health care and complex needs, the large volume of patients and their complexity may pose a challenge to the program, therefore having an additional tracking system in place may help mitigate possible losses to follow-up. In fact, since the implementation of tool, our team has been able to contact families and in some cases have reinstated services. As a by-product of tool implementation, we have implemented new policies to prevent extended losses to follow-up and patient attrition.
Limitations
A limitation of the assessment tool is that it does not incorporate the perspectives of the other stakeholders (adolescents, parents, adult providers). Further, some of the items in our tool are measuring utilization of services and not specifically transition readiness. As with most transition readiness measures, our provider tool does not have established reliability and validity [14]. We plan to test for reliability and validity once enough data and patient outcomes have been collected. Additionally, because of the small number of patients who have transferred to adult care since implementation of the tool, we did not examine the association between readiness scores and clinical outcomes, such as fulfillment of first adult provider visit and hospital utilization following transition to adult care. As we continue to assess adolescent patients and track their progress following transition, we will be able to examine these associations with a larger group.
Future Plans
Since the implementation of the tool in our program, we have realized that we may need to start assessing patients at an earlier age and perhaps multiple times throughout adolescence. Some of our patients have guardianship and conservatorship issues and require more time to discuss options with the family and put in place the appropriate support and assistance prior to the transfer of care. Further, patients that have low compliance to clinic appointments are not receiving all elements of the transition program curriculum and in turn have fewer opportunities to prepare for transition. To address some of our current limitations, we plan to incorporate a patient and parent readiness assessment and examine the associations between the provider assessment and patient information such as medical literacy quizzes, clinic compliance, and fulfillment of the first adult provider visit. Assessment from all 3 perspectives (patient, parent, and provider) will offer a 360-degree view of transition readiness perception which should improve our ability to identify at-risk families and tailor transition planning to address barriers to care. In addition, our future plans include development of a mechanism to inform patients and families about the domain scores and action plans following the transition readiness meetings and include scores into the electronic medical records. Finally, the readiness assessment tool has revealed some gaps in our transition educational curriculum. Most of our transition learning involves providing and evaluating information provided, but we are not systematically assessing actual acquired transition skills. We are in the process of developing and implementing skill-based learning for activities such as calling to make or reschedule an appointment with an adult provider, arranging transportation, etc.
Conclusion
In conclusion, the provider transition readiness assessment has been a helpful tool to monitor progress of adolescents with SCD towards readiness for transition. The QI methodology and PDSA cycle approach has not only allowed for testing, development, and implementation of the tool, but is also allowing ongoing systematic refinement of our instrument. This approach highlighted the psychosocial challenges of our families as they move toward the transfer of care, in addition to the need for more individualized planning. The next important step is to evaluate the validity and reliability of the measure so we can better evaluate the impact of transition programming on the transfer from pediatric to adult care. We found the PDSA cycle approach to be a framework that can efficiently and systematically improve the quality of care of transitioning patients with SCD and their families.
Corresponding author: Jerlym Porter, PhD, MPH, St. Jude Children’s Research Hosp., 262 Danny Thomas Pl., Mail stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02.
Financial disclosures: None.
1. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38(4 Suppl):S512–S521.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. Lanzkron S, Carroll CP, Haywood C, Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
5. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
6. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
7. Jordan L, Swerdlow P, Coates TD. Systematic review of transition from adolescent to adult care in patients with sickle cell disease. J Pediatr Hematol Oncol 2013;35:165–9.
8. McPherson M, Thaniel L, Minniti CP. Transition of patients with sickle cell disease from pediatric to adult care: assessing patient readiness. Pediatr Blood Cancer 2009;52:838–41.
9. Lebensburger JD, Bemrich-Stolz CJ, Howard TH. Barriers in transition from pediatrics to adult medicine in sickle cell anemia. J Blood Med 2012;3:105–12.
10. Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ--Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71.
11. Ferris ME, Harward DH, Bickford K, et al. A clinical tool to measure the components of health-care transition from pediatric care to adult care: the UNC TR(x)ANSITION scale. Ren Fail 2012;34:744–53.
12. Gilleland J, Amaral S, Mee L, Blount R. Getting ready to leave: transition readiness in adolescent kidney transplant recipients. J Pediatr Psychol 2012;37:85–96.
13. Cappelli M, MacDonald NE, McGrath PJ. Assessment of readiness to transfer to adult care for adolescents with cystic fibrosis. Child Health Care 1989;18:218–24.
14. Stinson J, Kohut SA, Spiegel L, et al. A systematic review of transition readiness and transfer satisfaction measures for adolescents with chronic illness. Int J Adolesc Med Health 2013:1–16.
15. Telfair J, Myers J, Drezner S. Transfer as a component of the transition of adolescents with sickle cell disease to adult care: adolescent, adult, and parent perspectives. J Adolesc Health 1994;15:558–65.
16. Walley P, Gowland B. Completing the circle: from PD to PDSA. Int J Health Care Qual Assur Inc Leadersh Health Serv 2004;17:349–58.
From the St. Jude Children’s Research Hospital, Memphis, TN.
This article is the fourth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the use of quality improvement (QI) methodology to implement an assessment tool to evaluate transition readiness in youth with sickle cell disease (SCD).
- Methods: Plan-Do-Study-Act (PDSA) cycles were run to evaluate the feasibility and effectiveness of a provider-based transition readiness assessment.
- Results: Seventy-two adolescents aged 17 years (53% male) were assessed for transition readiness from August 2011 to June 2013. Results indicated that it is feasible for a provider transition readiness assessment (PTRA) tool to be integrated into a transition program. The newly created PTRA tool can inform the level of preparedness of adolescents with SCD during planning for adult transition.
- Conclusion: The PTRA tool may be helpful for planning and preparation of youth with SCD to successfully transition to adult care.
Sickle cell disease (SCD) is one of the most common genetic disorders in the world and is caused by a mutation producing the abnormal sickle hemoglobin. Patients with SCD are living longer and transitioning from pediatric to adult providers. However, the transition years are associated with high mortality [1–4], risk for increased utilization of emergency care, and underutilization of care maintenance visits [5,6]. Successful transition from pediatric care to adult care is critical in ensuring care continuity and optimal health [7]. Barriers to successful transition include lack of preparation for transition [8,9]. To address this limitation, transition programs have been created to help foster transition preparation and readiness.
Often, chronological age determines when SCD programs transfer patients to adult care; however, age is an inadequate measure of readiness. To determine the appropriate time for transition and to individualize the subsequent preparation and planning prior to transfer, an assessment of transition readiness is needed. A number of checklists exist in the unpublished literature (eg, on institution and program websites), and a few empirically tested transition readiness measures have been developed through literature review, semi-structured interviews, and pilot testing in patient samples [10–13]. The Transition Readiness Assessment Questionnaire (TRAQ) and TRxANSITION scale are non-disease-specific measures that assess self-management and advocacy skills of youth with special health care needs; the TRAQ is self-report whereas the TRxANSITION scale is provider-administered [10,11]. Disease-specific measures have been developed for pediatric kidney transplant recipients [12] and adolescents with cystic fibrosis [13]. Studies using these measures suggest that transition readiness is associated with age, gender, disease type, increased adolescent responsibility/decreased parental involvement, and adherence [10–12].
For patients with SCD, there is no well-validated measure available to assess transition readiness [14]. Telfair and colleagues developed a sickle cell transfer questionnaire that focused on transition concerns and feelings and suggestions for transition intervention programming from the perspective of adolescents, their primary caregivers, and adults with SCD [15]. In addition, McPherson and colleagues examined SCD transition readiness in 4 areas: prior thought about transition, knowledge about steps to transition, interest in learning more about the transition process, and perceived importance of continuing care with a hematologist as an adult provider [8]. They found that adolescents in general were not prepared for transition but that readiness improved with age [8]. Overall, most readiness measures have involved patient self-report or parent proxy report. No current readiness assessment scales incorporate the provider’s assessment, which could help better define the most appropriate next steps in education and preparation for the upcoming transfer to adult care.
The St. Jude Children’s Research Hospital SCD Transition to Adult Care program was started in 2007 and is a companion program to the SCD teen clinic, serving 250 adolescents aged 12 to 18 years. The transition program curriculum addresses all aspects of the transition process. Based on the curriculum components, St. Jude developed and implemented a transition readiness assessment tool to be completed by providers in the SCD transition program. In this article, we describe our use of quality improvement (QI) methodology to evaluate the utility and impact of the newly created SCD transition readiness assessment tool.
Methods
Transition Program
The transition program is directed by a multidisciplinary team; disciplines represented on the team are medical (hematologist, genetic educator, physician assistant, and nurse coordinators), psychosocial (social workers), emotional/cognitive (psychologists), and academic (academic coordinator). In the program, adolescents with SCD and their families are introduced to the concept of transition to adult care at the age of 12. Every 6 months from 12 to 18 years of age, members of the team address relevant topics with patients to increase patients’ disease knowledge and improve their disease self-management skills. Some of the program components include training in completing a personal health record (PHR), genetic education, academic planning, and independent living skills.
Needs Assessment
Prior to initiation of the project, members of the transition program met monthly to informally discuss the progress of patients who were approaching the age of transition to adult care. We found that adolescents did not appear to be ready or well prepared for transition, including not being aware of the various familial and psychosocial issues that needed to be addressed prior to the transfer to adult care. We realized that these discussions needed to occur earlier to allow more time for preparation and transition planning of the patient, family, and medical team. In addition, members of the team each has differing perspectives and did not have the same information with regard to existing familial and psychosocial issues. The discussions were necessary to ensure all team members had pertinent information to make informed decisions about the patient’s level of transition readiness. Finally, our criteria for readiness were not standardized or quantifiable. As a result, each patient discussion was lengthy, not structured, and not very informative. In 2011, a core group from the transition team attended a Health Resources Services Administration–sponsored Hemoglobinopathies Quality Improvement Workshop to receive training in QI processes. We decided to create a formal, quantitative, individualized assessment of patients’ progress toward transition at age 17.
Readiness Assessment Tool
The emotional/cognitive domain checklist was developed by the pediatric psychologist and pediatric neuropsychologist. Because the psychology service is set up to see patients referred by the medical team and is unable to see all patients coming to hematology clinic, the emotional/cognitive checklist is based on identifying previous utilization of psychological services including psychotherapy and cognitive testing and determining whether initiation of services is warranted. The academic domain checklist was developed by the academic coordinator who serves as a liaison between the medical team and the school system. This checklist assesses whether the adolescent is meeting high school graduation requirements, able to verbalize an educational/job training plan, on track with future planning (eg, completed required testing), knowledgeable about community educational services, and able to self-advocate (eg, apply for SSI benefits).
Items within each domain have equal value (ie, each question on the checklist is worth 1 point) and the sum of points yields the quantifiable assessment of how well patients are performing in each area of their health. Assessment meetings occur monthly when eligible patients are discussed. Domains are evaluated by the health care provider responsible for his/her own domain (eg, social worker completes the psychosocial domain, the academic coordinator completes the academic domain, etc.).
PDSA Methodology
Cycle 1
The objective of the first cycle was to assess feasibility and acceptability of the assessment tool. Patients were assessed during the month of their 17th birthday. Fourteen out of 16 eligible patients (87.5%) were assessed: 1 patient was lost to follow-up, and 1 patient inadvertently was not included in the assessment due to an administrative error. Feedback from the first cycle revealed that some items on the emotional/cognitive domain checklist were not clearly defined, and there was some overlap with the psychosocial domain checklist. Additionally, some items were not readily assessed by psychology based on the structure of psychology services at the institution. Not all patients are seen by psychology; patients are referred to psychology by the team and appointments occur in the psychology clinic and were not well-integrated within the hematology clinic visit.
Cycle 2
The second cycle addressed some of the problems identified during Cycle 1. The emotional/cognitive domain checklist was revised to reflect psychology clinic utilization (psychotherapy and testing) and a section was added where team members could indicate individualized action plans. Seventeen patients out of 18 eligible patients were assessed (94.4%): 1 patient was lost to follow-up. At the conclusion of this cycle, we found that several patients had not completed certain transition program components, such as genetic education or their PHR. Therefore, we decided that we needed to indicate this and create a Plan of Action (POA) to ensure completion of program components. The POA indicated which components were outstanding, when these components would be completed, and when the team would discuss the patient again to track their progress with program components (eg, 6 months later).
Cycle 3
Following a few months using the assessment process, each member of the team provided feedback about their observations from the second cycle. The third cycle of the PDSA addressed some of the barriers identified in Cycle 2 by adding the POA and timeline for reassessment. With this information, the nurse case manager was able to identify and contact families who had significant gaps in the learning curriculum. Additionally, services such as psychological testing were scheduled in a timely manner to address academic problems and to provide rationale for accommodations and academic/vocational services before patients transferred care to the adult provider. With the number of assessed patients increasing, it was determined that a reliable tracking system to monitor progress was essential. Thus, a transition database was created to document the domain scores, individualized plan of action, and other components of the transition program, such as medical literacy quiz scores, completion of pre-transfer visits to adult providers, and completion of the PHR. During this cycle, 20 patients were assessed out of a total of 22 eligible patients (90.9%); 2 patients were lost to follow-up.
Cycle 4
This cycle is currently underway and comprises monthly assessments of eligible 17-year-old patients with SCD. From January 2013 to May 2013 we have assessed 100% of the eligible patients (21/21). All information obtained through the assessment tool is added to the transition database. Future adjustments and modifications are planned for this tool as we continue to evaluate its impact and value.
Discussion
The transition readiness assessment tool was developed to evaluate adolescent patients with SCD aged 17 years regarding their progress in the transition program and level of transition readiness. Most transition readiness measures available in the literature consider the patient and parent perspective but do not include the health care provider perspective or determine if the patient received the information necessary for successful transition. Our readiness assessment tool has been helpful in providing a structured and quantifiable means to identify at-risk patients and families prior to the transfer of care and revealing important gaps in transition planning. It also provides information in a timely manner about points of intervention to ensure patients receive adequate preparation and services (eg, psychological/neuropsychological testing). Additionally, monthly meetings are held during which the tool is scored and discussed, providing an opportunity for members of the transition team to examine patients’ progress toward transition readiness. Finally, completing an individualized tool in a multidisciplinary setting has the added benefit of encouraging increased staff collaboration and creating a venue for ongoing re-evaluation of the QI process.
We achieved our objective of completing the assessment tool for 80% of eligible patients throughout the cycles. The majority of our nonassessed patients was lost to follow-up and had not had a clinic visit in 2 to 3 years. Implementing the tool has provided us with an additional mechanism to verify transition eligibility and has afforded the transition program a systematic way to screen and track patients who are approaching the age of transition and who may have not been seen for an extended period of time. As with any large program following children with special health care and complex needs, the large volume of patients and their complexity may pose a challenge to the program, therefore having an additional tracking system in place may help mitigate possible losses to follow-up. In fact, since the implementation of tool, our team has been able to contact families and in some cases have reinstated services. As a by-product of tool implementation, we have implemented new policies to prevent extended losses to follow-up and patient attrition.
Limitations
A limitation of the assessment tool is that it does not incorporate the perspectives of the other stakeholders (adolescents, parents, adult providers). Further, some of the items in our tool are measuring utilization of services and not specifically transition readiness. As with most transition readiness measures, our provider tool does not have established reliability and validity [14]. We plan to test for reliability and validity once enough data and patient outcomes have been collected. Additionally, because of the small number of patients who have transferred to adult care since implementation of the tool, we did not examine the association between readiness scores and clinical outcomes, such as fulfillment of first adult provider visit and hospital utilization following transition to adult care. As we continue to assess adolescent patients and track their progress following transition, we will be able to examine these associations with a larger group.
Future Plans
Since the implementation of the tool in our program, we have realized that we may need to start assessing patients at an earlier age and perhaps multiple times throughout adolescence. Some of our patients have guardianship and conservatorship issues and require more time to discuss options with the family and put in place the appropriate support and assistance prior to the transfer of care. Further, patients that have low compliance to clinic appointments are not receiving all elements of the transition program curriculum and in turn have fewer opportunities to prepare for transition. To address some of our current limitations, we plan to incorporate a patient and parent readiness assessment and examine the associations between the provider assessment and patient information such as medical literacy quizzes, clinic compliance, and fulfillment of the first adult provider visit. Assessment from all 3 perspectives (patient, parent, and provider) will offer a 360-degree view of transition readiness perception which should improve our ability to identify at-risk families and tailor transition planning to address barriers to care. In addition, our future plans include development of a mechanism to inform patients and families about the domain scores and action plans following the transition readiness meetings and include scores into the electronic medical records. Finally, the readiness assessment tool has revealed some gaps in our transition educational curriculum. Most of our transition learning involves providing and evaluating information provided, but we are not systematically assessing actual acquired transition skills. We are in the process of developing and implementing skill-based learning for activities such as calling to make or reschedule an appointment with an adult provider, arranging transportation, etc.
Conclusion
In conclusion, the provider transition readiness assessment has been a helpful tool to monitor progress of adolescents with SCD towards readiness for transition. The QI methodology and PDSA cycle approach has not only allowed for testing, development, and implementation of the tool, but is also allowing ongoing systematic refinement of our instrument. This approach highlighted the psychosocial challenges of our families as they move toward the transfer of care, in addition to the need for more individualized planning. The next important step is to evaluate the validity and reliability of the measure so we can better evaluate the impact of transition programming on the transfer from pediatric to adult care. We found the PDSA cycle approach to be a framework that can efficiently and systematically improve the quality of care of transitioning patients with SCD and their families.
Corresponding author: Jerlym Porter, PhD, MPH, St. Jude Children’s Research Hosp., 262 Danny Thomas Pl., Mail stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02.
Financial disclosures: None.
From the St. Jude Children’s Research Hospital, Memphis, TN.
This article is the fourth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the use of quality improvement (QI) methodology to implement an assessment tool to evaluate transition readiness in youth with sickle cell disease (SCD).
- Methods: Plan-Do-Study-Act (PDSA) cycles were run to evaluate the feasibility and effectiveness of a provider-based transition readiness assessment.
- Results: Seventy-two adolescents aged 17 years (53% male) were assessed for transition readiness from August 2011 to June 2013. Results indicated that it is feasible for a provider transition readiness assessment (PTRA) tool to be integrated into a transition program. The newly created PTRA tool can inform the level of preparedness of adolescents with SCD during planning for adult transition.
- Conclusion: The PTRA tool may be helpful for planning and preparation of youth with SCD to successfully transition to adult care.
Sickle cell disease (SCD) is one of the most common genetic disorders in the world and is caused by a mutation producing the abnormal sickle hemoglobin. Patients with SCD are living longer and transitioning from pediatric to adult providers. However, the transition years are associated with high mortality [1–4], risk for increased utilization of emergency care, and underutilization of care maintenance visits [5,6]. Successful transition from pediatric care to adult care is critical in ensuring care continuity and optimal health [7]. Barriers to successful transition include lack of preparation for transition [8,9]. To address this limitation, transition programs have been created to help foster transition preparation and readiness.
Often, chronological age determines when SCD programs transfer patients to adult care; however, age is an inadequate measure of readiness. To determine the appropriate time for transition and to individualize the subsequent preparation and planning prior to transfer, an assessment of transition readiness is needed. A number of checklists exist in the unpublished literature (eg, on institution and program websites), and a few empirically tested transition readiness measures have been developed through literature review, semi-structured interviews, and pilot testing in patient samples [10–13]. The Transition Readiness Assessment Questionnaire (TRAQ) and TRxANSITION scale are non-disease-specific measures that assess self-management and advocacy skills of youth with special health care needs; the TRAQ is self-report whereas the TRxANSITION scale is provider-administered [10,11]. Disease-specific measures have been developed for pediatric kidney transplant recipients [12] and adolescents with cystic fibrosis [13]. Studies using these measures suggest that transition readiness is associated with age, gender, disease type, increased adolescent responsibility/decreased parental involvement, and adherence [10–12].
For patients with SCD, there is no well-validated measure available to assess transition readiness [14]. Telfair and colleagues developed a sickle cell transfer questionnaire that focused on transition concerns and feelings and suggestions for transition intervention programming from the perspective of adolescents, their primary caregivers, and adults with SCD [15]. In addition, McPherson and colleagues examined SCD transition readiness in 4 areas: prior thought about transition, knowledge about steps to transition, interest in learning more about the transition process, and perceived importance of continuing care with a hematologist as an adult provider [8]. They found that adolescents in general were not prepared for transition but that readiness improved with age [8]. Overall, most readiness measures have involved patient self-report or parent proxy report. No current readiness assessment scales incorporate the provider’s assessment, which could help better define the most appropriate next steps in education and preparation for the upcoming transfer to adult care.
The St. Jude Children’s Research Hospital SCD Transition to Adult Care program was started in 2007 and is a companion program to the SCD teen clinic, serving 250 adolescents aged 12 to 18 years. The transition program curriculum addresses all aspects of the transition process. Based on the curriculum components, St. Jude developed and implemented a transition readiness assessment tool to be completed by providers in the SCD transition program. In this article, we describe our use of quality improvement (QI) methodology to evaluate the utility and impact of the newly created SCD transition readiness assessment tool.
Methods
Transition Program
The transition program is directed by a multidisciplinary team; disciplines represented on the team are medical (hematologist, genetic educator, physician assistant, and nurse coordinators), psychosocial (social workers), emotional/cognitive (psychologists), and academic (academic coordinator). In the program, adolescents with SCD and their families are introduced to the concept of transition to adult care at the age of 12. Every 6 months from 12 to 18 years of age, members of the team address relevant topics with patients to increase patients’ disease knowledge and improve their disease self-management skills. Some of the program components include training in completing a personal health record (PHR), genetic education, academic planning, and independent living skills.
Needs Assessment
Prior to initiation of the project, members of the transition program met monthly to informally discuss the progress of patients who were approaching the age of transition to adult care. We found that adolescents did not appear to be ready or well prepared for transition, including not being aware of the various familial and psychosocial issues that needed to be addressed prior to the transfer to adult care. We realized that these discussions needed to occur earlier to allow more time for preparation and transition planning of the patient, family, and medical team. In addition, members of the team each has differing perspectives and did not have the same information with regard to existing familial and psychosocial issues. The discussions were necessary to ensure all team members had pertinent information to make informed decisions about the patient’s level of transition readiness. Finally, our criteria for readiness were not standardized or quantifiable. As a result, each patient discussion was lengthy, not structured, and not very informative. In 2011, a core group from the transition team attended a Health Resources Services Administration–sponsored Hemoglobinopathies Quality Improvement Workshop to receive training in QI processes. We decided to create a formal, quantitative, individualized assessment of patients’ progress toward transition at age 17.
Readiness Assessment Tool
The emotional/cognitive domain checklist was developed by the pediatric psychologist and pediatric neuropsychologist. Because the psychology service is set up to see patients referred by the medical team and is unable to see all patients coming to hematology clinic, the emotional/cognitive checklist is based on identifying previous utilization of psychological services including psychotherapy and cognitive testing and determining whether initiation of services is warranted. The academic domain checklist was developed by the academic coordinator who serves as a liaison between the medical team and the school system. This checklist assesses whether the adolescent is meeting high school graduation requirements, able to verbalize an educational/job training plan, on track with future planning (eg, completed required testing), knowledgeable about community educational services, and able to self-advocate (eg, apply for SSI benefits).
Items within each domain have equal value (ie, each question on the checklist is worth 1 point) and the sum of points yields the quantifiable assessment of how well patients are performing in each area of their health. Assessment meetings occur monthly when eligible patients are discussed. Domains are evaluated by the health care provider responsible for his/her own domain (eg, social worker completes the psychosocial domain, the academic coordinator completes the academic domain, etc.).
PDSA Methodology
Cycle 1
The objective of the first cycle was to assess feasibility and acceptability of the assessment tool. Patients were assessed during the month of their 17th birthday. Fourteen out of 16 eligible patients (87.5%) were assessed: 1 patient was lost to follow-up, and 1 patient inadvertently was not included in the assessment due to an administrative error. Feedback from the first cycle revealed that some items on the emotional/cognitive domain checklist were not clearly defined, and there was some overlap with the psychosocial domain checklist. Additionally, some items were not readily assessed by psychology based on the structure of psychology services at the institution. Not all patients are seen by psychology; patients are referred to psychology by the team and appointments occur in the psychology clinic and were not well-integrated within the hematology clinic visit.
Cycle 2
The second cycle addressed some of the problems identified during Cycle 1. The emotional/cognitive domain checklist was revised to reflect psychology clinic utilization (psychotherapy and testing) and a section was added where team members could indicate individualized action plans. Seventeen patients out of 18 eligible patients were assessed (94.4%): 1 patient was lost to follow-up. At the conclusion of this cycle, we found that several patients had not completed certain transition program components, such as genetic education or their PHR. Therefore, we decided that we needed to indicate this and create a Plan of Action (POA) to ensure completion of program components. The POA indicated which components were outstanding, when these components would be completed, and when the team would discuss the patient again to track their progress with program components (eg, 6 months later).
Cycle 3
Following a few months using the assessment process, each member of the team provided feedback about their observations from the second cycle. The third cycle of the PDSA addressed some of the barriers identified in Cycle 2 by adding the POA and timeline for reassessment. With this information, the nurse case manager was able to identify and contact families who had significant gaps in the learning curriculum. Additionally, services such as psychological testing were scheduled in a timely manner to address academic problems and to provide rationale for accommodations and academic/vocational services before patients transferred care to the adult provider. With the number of assessed patients increasing, it was determined that a reliable tracking system to monitor progress was essential. Thus, a transition database was created to document the domain scores, individualized plan of action, and other components of the transition program, such as medical literacy quiz scores, completion of pre-transfer visits to adult providers, and completion of the PHR. During this cycle, 20 patients were assessed out of a total of 22 eligible patients (90.9%); 2 patients were lost to follow-up.
Cycle 4
This cycle is currently underway and comprises monthly assessments of eligible 17-year-old patients with SCD. From January 2013 to May 2013 we have assessed 100% of the eligible patients (21/21). All information obtained through the assessment tool is added to the transition database. Future adjustments and modifications are planned for this tool as we continue to evaluate its impact and value.
Discussion
The transition readiness assessment tool was developed to evaluate adolescent patients with SCD aged 17 years regarding their progress in the transition program and level of transition readiness. Most transition readiness measures available in the literature consider the patient and parent perspective but do not include the health care provider perspective or determine if the patient received the information necessary for successful transition. Our readiness assessment tool has been helpful in providing a structured and quantifiable means to identify at-risk patients and families prior to the transfer of care and revealing important gaps in transition planning. It also provides information in a timely manner about points of intervention to ensure patients receive adequate preparation and services (eg, psychological/neuropsychological testing). Additionally, monthly meetings are held during which the tool is scored and discussed, providing an opportunity for members of the transition team to examine patients’ progress toward transition readiness. Finally, completing an individualized tool in a multidisciplinary setting has the added benefit of encouraging increased staff collaboration and creating a venue for ongoing re-evaluation of the QI process.
We achieved our objective of completing the assessment tool for 80% of eligible patients throughout the cycles. The majority of our nonassessed patients was lost to follow-up and had not had a clinic visit in 2 to 3 years. Implementing the tool has provided us with an additional mechanism to verify transition eligibility and has afforded the transition program a systematic way to screen and track patients who are approaching the age of transition and who may have not been seen for an extended period of time. As with any large program following children with special health care and complex needs, the large volume of patients and their complexity may pose a challenge to the program, therefore having an additional tracking system in place may help mitigate possible losses to follow-up. In fact, since the implementation of tool, our team has been able to contact families and in some cases have reinstated services. As a by-product of tool implementation, we have implemented new policies to prevent extended losses to follow-up and patient attrition.
Limitations
A limitation of the assessment tool is that it does not incorporate the perspectives of the other stakeholders (adolescents, parents, adult providers). Further, some of the items in our tool are measuring utilization of services and not specifically transition readiness. As with most transition readiness measures, our provider tool does not have established reliability and validity [14]. We plan to test for reliability and validity once enough data and patient outcomes have been collected. Additionally, because of the small number of patients who have transferred to adult care since implementation of the tool, we did not examine the association between readiness scores and clinical outcomes, such as fulfillment of first adult provider visit and hospital utilization following transition to adult care. As we continue to assess adolescent patients and track their progress following transition, we will be able to examine these associations with a larger group.
Future Plans
Since the implementation of the tool in our program, we have realized that we may need to start assessing patients at an earlier age and perhaps multiple times throughout adolescence. Some of our patients have guardianship and conservatorship issues and require more time to discuss options with the family and put in place the appropriate support and assistance prior to the transfer of care. Further, patients that have low compliance to clinic appointments are not receiving all elements of the transition program curriculum and in turn have fewer opportunities to prepare for transition. To address some of our current limitations, we plan to incorporate a patient and parent readiness assessment and examine the associations between the provider assessment and patient information such as medical literacy quizzes, clinic compliance, and fulfillment of the first adult provider visit. Assessment from all 3 perspectives (patient, parent, and provider) will offer a 360-degree view of transition readiness perception which should improve our ability to identify at-risk families and tailor transition planning to address barriers to care. In addition, our future plans include development of a mechanism to inform patients and families about the domain scores and action plans following the transition readiness meetings and include scores into the electronic medical records. Finally, the readiness assessment tool has revealed some gaps in our transition educational curriculum. Most of our transition learning involves providing and evaluating information provided, but we are not systematically assessing actual acquired transition skills. We are in the process of developing and implementing skill-based learning for activities such as calling to make or reschedule an appointment with an adult provider, arranging transportation, etc.
Conclusion
In conclusion, the provider transition readiness assessment has been a helpful tool to monitor progress of adolescents with SCD towards readiness for transition. The QI methodology and PDSA cycle approach has not only allowed for testing, development, and implementation of the tool, but is also allowing ongoing systematic refinement of our instrument. This approach highlighted the psychosocial challenges of our families as they move toward the transfer of care, in addition to the need for more individualized planning. The next important step is to evaluate the validity and reliability of the measure so we can better evaluate the impact of transition programming on the transfer from pediatric to adult care. We found the PDSA cycle approach to be a framework that can efficiently and systematically improve the quality of care of transitioning patients with SCD and their families.
Corresponding author: Jerlym Porter, PhD, MPH, St. Jude Children’s Research Hosp., 262 Danny Thomas Pl., Mail stop 740, Memphis, TN 38105, jerlym.porter@stjude.org.
Funding/support: This work was supported in part by HRSA grant 6 U1EMC19331-03-02.
Financial disclosures: None.
1. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38(4 Suppl):S512–S521.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. Lanzkron S, Carroll CP, Haywood C, Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
5. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
6. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
7. Jordan L, Swerdlow P, Coates TD. Systematic review of transition from adolescent to adult care in patients with sickle cell disease. J Pediatr Hematol Oncol 2013;35:165–9.
8. McPherson M, Thaniel L, Minniti CP. Transition of patients with sickle cell disease from pediatric to adult care: assessing patient readiness. Pediatr Blood Cancer 2009;52:838–41.
9. Lebensburger JD, Bemrich-Stolz CJ, Howard TH. Barriers in transition from pediatrics to adult medicine in sickle cell anemia. J Blood Med 2012;3:105–12.
10. Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ--Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71.
11. Ferris ME, Harward DH, Bickford K, et al. A clinical tool to measure the components of health-care transition from pediatric care to adult care: the UNC TR(x)ANSITION scale. Ren Fail 2012;34:744–53.
12. Gilleland J, Amaral S, Mee L, Blount R. Getting ready to leave: transition readiness in adolescent kidney transplant recipients. J Pediatr Psychol 2012;37:85–96.
13. Cappelli M, MacDonald NE, McGrath PJ. Assessment of readiness to transfer to adult care for adolescents with cystic fibrosis. Child Health Care 1989;18:218–24.
14. Stinson J, Kohut SA, Spiegel L, et al. A systematic review of transition readiness and transfer satisfaction measures for adolescents with chronic illness. Int J Adolesc Med Health 2013:1–16.
15. Telfair J, Myers J, Drezner S. Transfer as a component of the transition of adolescents with sickle cell disease to adult care: adolescent, adult, and parent perspectives. J Adolesc Health 1994;15:558–65.
16. Walley P, Gowland B. Completing the circle: from PD to PDSA. Int J Health Care Qual Assur Inc Leadersh Health Serv 2004;17:349–58.
1. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38(4 Suppl):S512–S521.
3. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60:1482–6.
4. Lanzkron S, Carroll CP, Haywood C, Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 2013;128:110–6.
5. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
6. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
7. Jordan L, Swerdlow P, Coates TD. Systematic review of transition from adolescent to adult care in patients with sickle cell disease. J Pediatr Hematol Oncol 2013;35:165–9.
8. McPherson M, Thaniel L, Minniti CP. Transition of patients with sickle cell disease from pediatric to adult care: assessing patient readiness. Pediatr Blood Cancer 2009;52:838–41.
9. Lebensburger JD, Bemrich-Stolz CJ, Howard TH. Barriers in transition from pediatrics to adult medicine in sickle cell anemia. J Blood Med 2012;3:105–12.
10. Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ--Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71.
11. Ferris ME, Harward DH, Bickford K, et al. A clinical tool to measure the components of health-care transition from pediatric care to adult care: the UNC TR(x)ANSITION scale. Ren Fail 2012;34:744–53.
12. Gilleland J, Amaral S, Mee L, Blount R. Getting ready to leave: transition readiness in adolescent kidney transplant recipients. J Pediatr Psychol 2012;37:85–96.
13. Cappelli M, MacDonald NE, McGrath PJ. Assessment of readiness to transfer to adult care for adolescents with cystic fibrosis. Child Health Care 1989;18:218–24.
14. Stinson J, Kohut SA, Spiegel L, et al. A systematic review of transition readiness and transfer satisfaction measures for adolescents with chronic illness. Int J Adolesc Med Health 2013:1–16.
15. Telfair J, Myers J, Drezner S. Transfer as a component of the transition of adolescents with sickle cell disease to adult care: adolescent, adult, and parent perspectives. J Adolesc Health 1994;15:558–65.
16. Walley P, Gowland B. Completing the circle: from PD to PDSA. Int J Health Care Qual Assur Inc Leadersh Health Serv 2004;17:349–58.
Using Quality Improvement Methods to Implement an Individualized Home Pain Management Plan for Children with Sickle Cell Disease
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
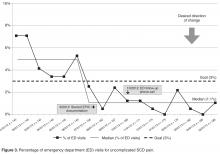
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
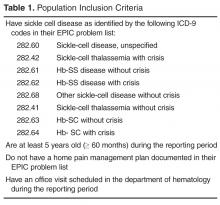
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
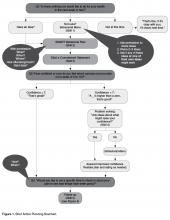
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
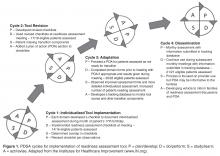
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
Brief Action Planning to Facilitate Behavior Change and Support Patient Self-Management
From the New York University School of Medicine, New York, NY (Drs. Gutnick and Jay), University of Colorado Health Sciences Center, Denver, CO (Dr. Reims), University of British Columbia, BC, Canada (Dr. Davis), University College London, London, UK (Dr. Gainforth), and Stonybrook University School of Medicine, Stonybrook, NY (Dr. Cole [Emeritus]).
Abstract
- Objective: To describe Brief Action Planning (BAP), a structured, stepped-care self-management support technique for chronic illness care and disease prevention.
- Methods: A review of the theory and research supporting BAP and the questions and skills that comprise the technique with provision of a clinical example.
- Results: BAP facilitates goal setting and action planning to build self-efficacy for behavior change. It is grounded in the principles and practice of Motivational Interviewing and evidence-based constructs from the behavior change literature. Comprised of a series of 3 questions and 5 skills, BAP can be implemented by medical teams to help meet the self-management support objectives of the Patient-Centered Medical Home.
- Conclusion: BAP is a useful self-management support technique for busy medical practices to promote health behavior change and build patient self-efficacy for improved long-term clinical outcomes in chronic illness care and disease prevention.
Chronic disease is prevalent and time consuming, challenging, and expensive to manage [1]. Half of all adult primary care patients have more than 2 chronic diseases, and 75% of US health care dollars are spent on chronic illness care [2]. Given the health and financial impact of chronic disease, and recognizing that patients make daily decisions that affect disease control, efforts are needed to assist and empower patients to actively self-manage health behaviors that influence chronic illness outcomes. Patients who are supported to actively self-manage their own chronic illnesses have fewer symptoms, improved quality of life, and lower use of health care resources [3]. Historically, providers have tried to influence chronic illness self-management by advising behavior change (eg, smoking cessation, exercise) or telling patients to take medications; yet clinicians often become frustrated when patients do not “adhere” to their professional advice [4,5]. Many times, patients want to make changes that will improve their health but need support—commonly known as self-management support—to be successful.
Involving patients in decision making, emphasizing problem solving, setting goals, creating action plans (ie, when, where and how to enact a goal-directed behavior), and following up on goals are key features of successful self-management support methods [3,6–8]. Multiple approaches from the behavioral change literature, such as the 5 A’s (Assess, Advise, Agree, Assist, Arrange) [9], Motivational Interviewing (MI), and chronic disease self-management programs [10] have been used to provide more effective guidance for patients and their caregivers. However, the practicalities of these approaches in clinical settings have been questioned. The 5A’s, a counseling framework that is used to guide providers in health behavior change counseling, can feel overwhelming because it encompasses several different aspects of counseling [11,12]. Likewise, MI and adaptations of MI, which have been shown to outperform traditional “advice giving” in treatment of a broad range of behaviors and chronic conditions [13–16], have been critiqued since fidelity to this approach often involves multiple sessions of training, practice, and feedback to achieve proficiency [15,17,18]. Finally, while chronic disease self-management programs have been shown to be effective when used by peers in the community [10], similar results in primary care are not well established.
Given the challenges of providers practicing, learning, and using each of these approaches, efforts to develop an approach that supports patients to make behavioral changes that can be implemented in typical practice settings are needed. In addition, health delivery systems are transforming to team-based models with emphasis on leveraging each team member’s expertise and licensure [19]. In acknowledgement of these evolving practice realities, the National Committee for Quality Assurance (NCQA) included development and documentation of patient self-management plans and goals as a critical factor for achieving NCQA Patient-Centered Medical Home (PCMH) recognition [20]. Successful PCMH transformation therefore entails clinical practices developing effective and time efficient ways to incorporate self-management support strategies, a new service for many, into their care delivery systems often without additional staffing.
In this paper, we describe an evidence-informed, efficient self-management support technique called Brief Action Planning (BAP) [21–24]. BAP evolved into its current form through ongoing collaborative efforts of 4 of the authors (SC, DG, CD, KR) and is based on a foundation of original work by Steven Cole with contributions from Mary Cole in 2002 [25]. This technique addresses many of the barriers providers have cited to providing self-management support, as it can be used routinely by both individual providers and health care teams to facilitate patient-centered goal setting and action planning. BAP integrates principles and practice of MI with goal setting and action planning concepts from the self-management support, self-efficacy, and behavior change literature. In addition to reviewing the principles and theory that inform BAP, we introduce the steps of BAP and discuss practical considerations for incorporating BAP into clinical practice. In particular, we include suggestions about how BAP can be used in team-based clinical practice settings within the PCMH. Finally, we present a common clinical scenario to demonstrate BAP and provide resource links to online videos of BAP encounters. Throughout the paper, we use the word “clinician” to refer to professionals or other trained personnel using BAP, and “patient” to refer to those experiencing BAP, recognizing that other terms may be preferred in different settings.
What is BAP?
BAP is a highly structured, stepped-care, self-management support technique. Composed of a series of 3 questions and 5 skills (reviewed in detail below), BAP can be used to facilitate goal setting and action planning to build self-efficacy in chronic illness management and disease prevention [21–24]. The overall goal of BAP is to assist an individual to create an action plan for a self-management behavior that they feel confident that they can achieve. BAP is currently being used in diverse care settings including primary care, home health care, rehabilitation, mental health and public health to assist and empower patients to self-manage chronic illnesses and disabilities including diabetes, depression, spinal cord injury, arthritis, and hypertension. BAP is also being used to assist patients to develop action plans for disease prevention. For example, the Bellevue Hospital Personalized Prevention clinic, a pilot clinic that uses a mathematical model [26] to help patients and providers collaboratively prioritize prevention focus and strategies, systematically utilizes BAP as its self-management support technique for patient-centered action planning. At this time, BAP has been incorporated into teaching curriculums at multiple medical schools, presented at major national health care/academic conferences and is being increasingly integrated into health delivery systems across the United States and Canada to support patient self-management for NCQA-PCMH transformation. We have also developed a series of standardized programing to support fidelity in BAP skills development including a multidisciplinary introductory training curriculum, telephonic coaching, interactive web-based training tools, and a structured “Train the Trainer” curriculum [27]. In addition, a set of guidelines designed to ensure fidelity in BAP research has been developed [27].
Underlying Principles of BAP
BAP is grounded in the principles and practice of MI and the psychology of behavior change. Within behavior change, we draw primarily on self-efficacy and action planning theory and research. We discuss the key concepts in detail below.
The Spirit of MI
MI Spirit (Compassion, Acceptance, Partnership and Evocation) is an important overarching tenet for BAP. Compassionately supporting self-management with MI spirit involves a partnership with the patient rather than a prescription for change and the assurance that the clinician has the patients best interest always in mind (Compassion) [17]. Exemplifying “spirit” accepts that the ultimate choice to change is the patient’s alone (Acceptance) and acknowledges that individuals bring expertise about themselves and their lives to the conversation (Evocation). Adherence to “MI spirit” itself has been associated with positive behavior change outcomes in patients [5,28–32]. Demonstrating MI spirit throughout the change conversation is an essential foundational principle of BAP.
Action Planning and Self-Efficacy
In addition to the spirit of MI, BAP integrates 2 evidence-based constructs from the behavior change literature: action planning and self-efficacy [4,6,33–36]. Action planning requires that individuals specify when, where and how to enact a goal-directed behavior (eg, self-management behaviors). Action planning has been shown to mediate the intention-behavior relationship thereby increasing the likelihood that an individual’s intentions will lead to behavior change [37,38]. Given the demonstrated potential of action planning for ensuring individuals achieve their health goals, the BAP framework aspires to assist patients to create an action plan.
BAP also aims to build patients’ self-efficacy to enact the goals outlined in their action plans. Self-efficacy refers to a patient’s confidence in their ability to enact a behavior [33]. Several reviews of the literature have suggested a strong relationship between self-efficacy and adoption of healthy behaviors such as smoking cessation, weight control, contraception, alcohol abuse and physical activity [39–42]. Furthermore, Lorig et al demonstrated that the process of action planning itself contributes to enhanced self-efficacy [8]. BAP aims to build self-efficacy and ultimately change patients’ behaviors by helping patients to set an action plan that they feel confident in their ability to achieve.
Description of the BAP Steps
Three questions and 3 of the BAP skills (ie, SMART plan, eliciting a commitment statement, and follow-up) are applied during every BAP interaction, while 2 skills (ie, behavioral menu and problem solving for low confidence) are used as needed. The distinct functions and the evidence supporting the 3 questions and 5 BAP skills are described below.
Question 1: Eliciting a Behavioral Focus or Goal
Once engagement has been established and the clinician determines the patient is ready for self-management planning to occur, the first question of BAP can be asked: “Is there anything you would like to do for your health in the next week or two?”
Although technically Question 1 is a closed-ended question (in that it can be answered “yes” or “no”), in actual practice it generates productive discussions about change.
1) Have an Idea. A group of patients immediately present an idea that they are ready to do or are ready to consider doing. For these patients, clinicians can proceed directly to Skill 2—SMART Behavioral Planning; that is, asking patients directly if they are ready to turn their idea into a concrete plan. Some evidence suggests that further discussion, assessment, or even additional "motivational" exploration in patients who are ready to make a plan and already have an idea may actually decrease motivation for change [17, 32].
2) Not Sure. Another group of patients may want or need suggestions before committing to something specific they want to work on. For these patients, clinicians should use the opportunity to offer a Behavioral Menu (Skill 1).
3) No or Not at This Time. A third group of patients may not be interested or ready to make a change at this time or at all. Some in this group may be healthy or already self-managing effectively and have no need to make a plan, in which case the clinician acknowledges their active self-management and moves to the next part of the visit. Others in this group may have considerable ambivalence about change or face complex situations where other priorities take precedence. Clinicians frequently label these individuals as "resistant." The Spirit of MI can be very useful when working with these patients to accept and respect their autonomy while encouraging ongoing partnership at a future time. For example, a clinician may say “It sounds like you are not interested in making a plan for your health right now. Would it be OK if I ask you about this again at our next visit?” Pushing forward to make a "plan for change" when a patient is not ready decreases both motivation for change as well as the likelihood for a successful outcome [32].
Other patients may benefit from additional motivational approaches to further explore change and ambivalence. If the clinician does not have these skills, patients may be seamlessly transitioned to another resource within or external to the care team.
Skill 1: Offering a Behavioral Menu
If in response to Question 1 an individual is unable to come up with an idea of their own or needs more information, then offering a Behavioral Menu may be helpful [44,45]. Consistent with the “Spirit of MI,” BAP attempts to elicit ideas from the individual themselves; however, it is important to recognize that some people require assistance to identify possible actions. A behavioral menu is comprised of 2 or 3 suggestions or ideas that will ideally trigger individuals to discover an idea of their own. There are 3 distinct evidence-based steps to follow when presenting a Behavioral Menu.
1) Ask permission to offer a behavioral menu. Asking permission to share ideas respects patient autonomy and prevents the provider from inadvertently assuming an expert role. For example: “Would it be OK if I shared with you some examples of what some other patients I work with have done?”
2) Offer 2 to 3 general yet varied ideas all at once (Figure 2, entry 5). It helps to mention things that other patients have decided to do with some success. Using this approach avoids the clinician assuming too much about the patient or allowing the patient to reject the ideas. It is important to remember that the list is to prompt ideas, not to find a perfect solution [17]. For example: “One patient I work with decided to join a gym and start exercising, another decided to pick up an old hobby he used to enjoy doing and another patient decided to schedule some time with a friend she hadn’t seen in a while.”
3) Ask if any of the ideas appeal to the individual as something that might work for them or if the patient has an idea of his/her own (Figure 2, entry 5). Evocation from the Spirit of MI is built in with this prompt [17]. For example: “These are some ideas that have worked for other patients I work with, do they trigger any ideas that might work for you?”
Skill 2: SMART Planning
Once an individual decides on an area of focus, the clinician partners with the patient to clarify the details and create an action plan to achieve their goal. Given that individuals are more likely to successfully achieve goals that are specific, proximal, and achievable as opposed to vague and distal [46,47], the clinician works with patient to ensure that the patient’s goal is SMART (specific, measurable, achievable, relevant and time-bound). The term SMART has its roots in the business management literature [48] as an adaptation of Locke’s pioneering research (1968) on goal setting and motivation [49]. In particular, Locke and Latham’s theory of Goal Setting and Task performance, states that “specific and achievable” goals are more likely to be successfully reached [47,50].
We suggest helping the patient to make smart goals by eliciting answers to questions applicable to the plan, such as “what?” “where?” “when?” “how long?” “how often?” “how much?” and “when will you start?” [51]. A resulting plan might be “I will walk for 20 minutes, in my neighborhood, every Monday, Wednesday and Friday before dinner.”
Skill 3: Elicit a Commitment Statement
Once the individual has developed a specific plan, the next step of BAP is for the clinician to ask him or her to “tell back” the specifics of the plan. The provider might say something like, “Just to make sure we understand each other, would you repeat back what you’ve decided to do?” The act of “repeating back” organizes the details of the plan in the persons mind and may lead to an unconscious self-reflection about the feasibility of the plan [43,52], which then sets the stage for Question 2 of BAP (Scaling for Confidence). Commitment predicts subsequent behavior change, and the strength of the commitment language is the strongest predictor of success on an action plan [43,52,53]. For example saying “I will” is stronger than saying “I will try.”
Question 2: Scaling for Confidence
After a commitment statement has been elicited, the second question of BAP is asked. “How confident or sure do you feel about carrying out your plan on a scale from 0 to 10, where 0 is not confident at all and 10 is totally confident or sure?” Confidence scaling is a common tool used in behavioral interventions, MI, and chronic disease self-management programs [17,51]. Question 2 assesses an individual’s self-efficacy to complete the plan and facilitates discussion about potential barriers to implementation in order to increase the likelihood of success of a personal action plan.
For patients who have difficulty grasping the concept of a numerical scale, the word “sure” can be substituted for “confident” and a Likert scale including the terms “not at all sure,” “somewhat sure,” and “very sure” substituted for the numerical confidence ruler, ie, “How sure are you that you will be able to carry out your plan? Not at all sure, somewhat sure, or very sure?” Alternatively, people of different cultural backgrounds may find it easier to grasp the concept using familiar images or experiences. For example, Native Americans from the Southwest have adapted the scale to depict a series of images ranging from planting a corn seed to harvesting a crop or climbing a ladder, while in some Latino cultures the image of climbing a mountain (“How far up the mountain are you?”) is useful to demonstrate “level of confidence” concept [54].
Skill 4: Problem Solving for Low Confidence
When confidence is relatively low (ie, below 7), we suggest collaborative problem solving as the next step [8,51]. Low confidence or self-efficacy for plan completion is a concern since low self-efficacy predicts non-completion [8]. Successfully implementing the action plan, no matter how small, increases confidence and self-efficacy for engaging in the behavior [8].
There are several steps that a clinician follows when collaboratively problem-solving with a patient with low confidence (Figure 1).
• Recognize that a low confidence level is greater than no confidence at all. By affirming the strength of a patient’s confidence rather than negatively focusing on a low level of confidence, the provider emphasizes the patient’s strengths.
• Collaboratively explore ways that the plan could be modified in order to improve confidence. A Behavioral Menu can be offered if needed. For example, a clinician might say something like: “That’s great that your confidence level is a 5. A 5 is a lot higher than a 1. People are more likely to have success with their action plans when confidence levels are 7 or more. Do you have any ideas of how you might be able to increase your level confidence to a 7 or more?”
• If the patient has no ideas, ask permission to offer a Behavioral Menu: “Would it be ok to share some ideas about how other patients I’ve worked with have increased their confidence level?” If the patient agrees, then say... “Some people modify their plans to make them easier, some choose a less ambitious goal or adjust the frequency of their plan, and some people involve a friend or family member. Perhaps one of these ideas seems like a good one for you or maybe you have another idea?”
Question 3: Arranging Accountability
Once the details of the plan have been determined and confidence level for success is high, the next step is to ask Question 3: “Would you like to set a specific time to check in about your plan to see how things are going?” This question encourages a patient to be accountable for their plan, and reinforces the concept that the physician and care team consider the plan to be important. Research supports that people are more likely to follow through with a plan if they choose to report back their progress [43] and suggests that checking-in frequently earlier in the process is helpful [55]. Ideally the clinician and patient should agree on a time to check in on the plan within a week or two (Figure 2, entry 29).
Accountability in the form of a check-in may be arranged with the clinical provider, another member of the healthcare team or a support person of the patient’s choice (eg, spouse, friend). The patient may also choose to be accountable to themselves by using a calendar or a goal setting application on their smart phone device or computer.
Skill 5: Follow-up
The purpose of the check-in is for learning and adjustment of the plan as well as to provide support regardless of outcome. Checking-in encourages reflection on challenges and barriers as well as successes. Patients should be given guidance to think through what worked for them and what did not. Focusing just on “success” of the plan will be less helpful. If follow-up is not done with the care team in the near term, checking-in can be accomplished at the next scheduled visit. Patient portals provide another opportunity for patients to dialogue with the care team about their plan.
Experiential Insights from Clinical Experience Using BAP
The authors collective experience to date indicates that between 50% to 75% of individuals who are asked Question 1 go on to develop an action plan for change with relatively little need for additional skills. In other studies of action planning in primary care, 83% of patients made action plans during a visit, and at 3-week follow-up 53% had completed their action plan [56]. A recent study of action planning using an online self-management support program reported that action plans were successfully completed (49%), partially completed (40%) or incomplete (11% of the time) [35].
Another caveat to consider is that the process of planning is more important that the actual plan itself. It is imperative to allow the patient, not the clinician, to determine the plan. For example, a patient with multiple poorly controlled chronic illnesses including depression may decide to focus his action plan around cleaning out his car rather than disease control such as dietary modification, medication adherence or exercise. The clinician may initially fail to view this as a good use of clinician time or healthcare resources since it seems unrelated to health. However, successful completion of an action plan is not the only objective of action planning. Building self-efficacy, which may lead to additional action planning around health, is more important [4,46]. The challenge is therefore for the clinician to take a step back, relinquish the “expert role,” and support the goal setting process regardless of the plan. In this example, successfully cleaning out his car may increase the patient’s self-efficacy to control other aspects of his life including diet and the focus of future plans may shift [4].
When to Use BAP
Opportunities for patient engagement in action planning occur when addressing chronic illness concerns as well as during discussions about health maintenance and preventive care. BAP can be considered as part of any routine clinical agenda unless patient preferences or clinical acuity preclude it. As with most clinical encounters, the flow is often negotiated at the beginning of the visit. BAP can be accomplished at any time that works best for the flow and substance of the visit, but a few patterns have emerged based on our experience.
BAP fits naturally into the part of the visit when the care plan is being discussed. The term “care plan” is commonly used to describe all of the care that will be provided until the next visit. Care plans can include additional recommendations for testing or screening, therapeutic adjustments and or referrals for additional expertise. Ideally the patients “agreed upon” contribution to their care should also be captured and documented in their care plan. This is often described as the patients “self-management goal.” For patients who are ready to make a specific plan to change behavior, BAP is an efficient way to support patients to craft an action plan that can then be incorporated into the overall care plan.
Another variation of when to use BAP is the situation when the patient has had a prior action plan and is being seen for a recheck visit. Discussing the action plan early in the visit agenda focuses attention on the work patients have put into following their plan. Descriptions of success lead readily to action plans for the future. Time spent discussing failures or partial success is valuable to problem solve as well as to affirm continued efforts to self-manage.
BAP can also be used between scheduled visits. The check-in portion of BAP is particularly amenable to follow-up by phone or by another supporter. A pre-arranged follow-up 1 to 2 weeks after creation of a new action plan [8] provides encouragement to patients working on their plan and also helps identify those who need more support.
Finally, BAP can be completed over multiple visits. For patients who are thinking about change but are not yet committed to planning, a brief suggestion about the value of action planning with a behavioral menu may encourage additional self-reflection. Many times patients return to the next visit with clear ideas about changes that would be important for them to make.
Fitting BAP into a 20-Minute Visit
Using BAP is a time-efficient way to provide self-management support within the context of a 20-minute visit with engaged patients who are ready to set goals for health. With practice, clinicians can often conduct all the steps within 3 to 5 minutes. However, patients and clinicians often have competing demands and agendas and may not feel that they have time to conduct all the steps. Thus, utilizing other members of the health care team to deliver some or all of BAP can facilitate implementation.
Teams have been creative in their approach to BAP implementation but 2 common models involve a multidisciplinary approach to BAP. In one model, the clinician assesses the patient readiness to make a specific action plan by asking Question 1, usually after the current status of key problems have been addressed and discussions begin about the interim plan of care. If the patient indicates interest, another staff member trained in BAP, such as an medical assistant, health coach or nurse, guides the development of the specific plan, completes the remaining steps and inputs the patient’s BAP into the care plan.
In another commonly deployed model, the front desk clerk or medical assistant helps to get the patient thinking by asking Question 1 and perhaps by providing a behavioral menu. When the clinician sees the patient, he follows up on the behavior change the patient has chosen and affirms the choice. Clinicians often flex seamlessly with other team members to complete the action plan depending on the schedule and current patient flow.
Regardless of how the workflows are designed, BAP implementation requires staff that can provide BAP with fidelity, effective communication among team members involved in the process and a standardized approach to documentation of the specific action plan, plan for check-in and notes about follow-up. Care teams commonly test different variations of personnel and workflows to find what works best for their particular practice.
Implementing BAP to Support PCMH Transformation
To support PCMH transformation substantial changes are needed to make care more proactive, more patient-centered and more accountable. One of the common elements for PCMH recognition regardless of sponsor is to enhance self-management support [20,57,58]. Practices pursuing PCMH designation are searching for effective evidence-based approaches to provide self-management support and guide action planning for patients. The authors suggest implementation of BAP as a potential strategy to enhance self-management support. In addition to facilitating meeting the actual PCMH criteria, BAP is aligned with the transitions in care delivery that are an important part of the transformation including reliance on team-based care and meaningful engagement of patients in their care [59,60].
In our experience, BAP is introduced incrementally into a practice initially focusing on one or two patient segments and then including more as resources allow. Successful BAP implementation begins with an organizational commitment to self-management support, decisions about which populations would benefit most from self-management support and BAP, training of key staff and clearly defined workflows that ensure reliable BAP provision.
BAP’s stepped-care design makes it easy to teach to all team members and as described above, team-based delivery of BAP functions well in those situations where clinicians and trained ancillary staff can “hand off” the process at any time to optimize the value to the patient while respecting inherent time constraints.
Documentation of the actual goal and follow-up is an important component to fully leverage BAP. Goals captured in a template generate actionable lists for action plan follow-up. Since EHRs vary considerably in their capacity to capture goals, teams adding BAP to their workflow will benefit from discussion of standardized documentation practices and forms.
Summary
Brief Action Planning is a self-management support technique that can be used in busy clinical settings to support patient self-management through patient-centered goal setting. Each step of BAP is based on principles grounded in evidence. Health care teams can learn BAP and integrate it into clinical delivery systems to support self-management for PCMH transformation.
Corresponding author: Damara Gutnick, MD, New York University School of Medicine, New York, NY, damaragutnick@gmail.com.
Financial disclosures: None.
1. Hoffman C, Rice D, Sung HY. Persons withnic conditions. Their prevalence and costs. JAMA 1996;276(18):1473–9.
2. Institute of Medicine. Living well with chro:ic illness: a call for public health action. Washington (DC); The National Academies Press; 2012.
3. De Silva D. Evidence: helping people help themselves. London: The Health Foundation Inspiring Improvement; 2011.
4. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–75.
5. Miller W, Benefield R, Tonigan J. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. J Consul Clin Psychol 1993;61:455–461.
6. Lorig K, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7.
7. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation 2010;122:406–41.
8. Lorig K, Laurent DD, Plant K, Krishnan E, Ritter PL. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013. Available at www.ncbi.nlm.nih.gov/pubmed/23838837.
9. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high-quality obesity counseling in primary care using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
10. Lorig KR, Ritter P, Stewart a L, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
11. Jay MR, Gillespie CC, Schlair SL, et al. The impact of primary care resident physician training on patient weight loss at 12 months. Obesity 2013;21:45–50.
12. Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med 2004;27:61–79.
13. Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93:157–68.
14. Rubak S, Sandbæk A, Lauritzen T, Christensen B. Motivational Interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–12.
15. Dunn C, Deroo L, Rivara F. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725–42.
16. Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control 2010;19:410–6.
17. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York: Guilford Press; 2013.
18. Resnicow K, DiIorio C, Soet J, et al. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol 2002;21:444–451.
19. Doherty RB, Crowley RA. Principles supporting dynamic clinical care teams: an American College of Physicians position paper. Ann Intern Med 2013;159:620–6.
20. NCQA PCMH 2011 Standards, Elements and Factors. Documentation Guideline/Data Sources. 4A: Provide self-care support and community resources. Available at www.ncqa.org/portals/0/Programs/Recognition/PCMH_2011_Data_Sources_6.6.12.pdf.
21. Reims K, Gutnick D, Davis C, Cole S. Brief action planning white paper. 2012. Available at www.centrecmi.ca.
22. Cole S, Davis C, Cole M, Gutnick D. Motivational interviewing and the patient centered medical home: a strategic approach to self-management support in primary care. In: Patient-Centered Primary Care Collaborative. Health IT in the patient centered medical home. October 2010. Available at www.pcpcc.net/guide/health-it-pcmh.
23. Cole S, Cole M, Gutnick D, Davis C. Function three: collaborate for management. In: Cole S, Bird J, editors. The medical interview: the three function approach. 3rd ed. Philadelphia:Saunders; 2014.
24. Cole S, Gutnick D, Davis C, Cole M. Brief action planning (BAP): a self-management support tool. In: Bickley L. Bates’ guide to physical examination and history taking. 11th ed. Philadelphia: Lippincott Williams and Wilkins; 2013.
25. AMA Physician tip sheet for self-management support. Available at www.ama-assn.org/ama1/pub/upload/mm/433/phys_tip_sheet.pdf.
26. Taksler G, Keshner M, Fagerlin A. Personalized estimates of benefit from preventive care guidelines. Ann Intern Med 2013;159:161–9.
27. Centre for Comprehensive Motivational Interventions [website]. Available at www.centreecmi.com.
28. Del Canale S, Louis DZ, Maio V, et al. The relationship between physician empathy and disease complications: an empirical study of primary care physicians and their diabetic patients in Parma, Italy. Acad Med 2012;87:1243–9.
29. Moyers TB, Miller WR, Hendrickson SML. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. J Consult Clin Psychol 2005;73:590–8.
30. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
31. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
32. Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37:129–40.
33. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;85:191–215.
34. Kiesler, Charles A. The psychology of commitment: experiments linking behavior to belief. New York: Academic Press;1971.
35. Lorig K, Laurent DD, Plant K, et al. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013.
36. MacGregor K, Handley M, Wong S, et al. Behavior-change action plans in primary care: a feasibility study of clinicians. J Am Board Fam Med 19:215–23.
37. Gollwitzer P. Implementation intentions. Am Psychol 1999;54:493–503.
38. Gollwitzer P, Sheeran P. Implementation intensions and goal achievement: A meta-analysis of effects and processes. Adv Exp Soc Psychology 2006;38:69–119.
39. Stretcher V, De Vellis B, Becker M, Rosenstock I. The role of self-efficacy in achieving behavior change. Health Educ Q 1986;13:73–92.
40. Ajzen I. Constructing a theory of planned behavior questionnaire. Available at people.umass.edu/aizen/pdf/tpb.measurement.pdf.
41. Rogers RW. Protection motivation theory of fear appeals and attitude-change. J Psychol 1975;91:93–114.
42. Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol An Int Rev 2008;57:1–29.
43. Cialdini R. Influence: science and practice. 5th ed. Boston:Allyn and Bacon; 2008.
44. Stott NC, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: diabetes care and negotiating skills. Fam Pract 1995;12:413–8.
45. Miller WR, Rollnick S, Butler C. Motivational interviewing in health care. New York: Guilford Press; 2008.
46. Bodenheimer T, Handley M. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns 2009;76:174–80.
47. Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. Am Psychol 2002;57:705–17.
48. Doran G. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev 1981;70:35–6.
49. Locke EA. Toward a theory of task motivation and incentives. Organ Behav Hum Perform 1968;3:157–89.
50. Locke EA, Latham GP, Erez M. The determinants of goal commitment. Acad Manag Rev 1988;13:23–39.
51. Lorig K, Homan H, Sobel D, et al. Living a healthy life with chronic conditions. 4th ed. Boulder: Bull Publishing; 2012.
52. Amrhein PC, Miller WR, Yahne CE, et al. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862–78.
53. Ahaeonovich E, Amrhein PC, Bisaha A, et al. Cognition, commitment language and behavioral change among cocaine-dependent patients. Psychol Addict Behav 2008;22:557–62.
54. Gutnick D. Centre for Comprehensive Motivational Interventions community of practice webinar. Brief action planning and culture: developing culturally specific confidence rules. 2012. Available at www.centrecmi.ca.
55. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults. A scientific statement from the American Heart Association. Circulation 2010;122:406–41.
56. Handley M, MacGregor K, Schillinger D, et al. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med 2006;19:224–31.
57. Joint Commision. Primary care medical home option-additional requirements. Available at www.jointcommission.org/assets/1/18/PCMH_new_stds_by_5_characteristics.pdf.
58. Oregon Health Policy and Research. Standards for patient centered medical home recognition. Available at www.oregon.gov/oha/OHPR/pages/healthreform/pcpch/standards.aspx.
59. Nutting PA, Crabtree BF, Miller WL, et al. Journey to the patient-centered medical home: a qualitative analysis of the experiences of practices in the national demonstration project. Am Fam Med 2010;8(Suppl 1):S45–S56.
60. Stewart EE, Nutting PA, Crabtree BF, et al. Implementing the patient-centered medical home: observation and description of the National Demonstration Project. Am Fam Med 2010;8(Suppl 1):S21–S32.
From the New York University School of Medicine, New York, NY (Drs. Gutnick and Jay), University of Colorado Health Sciences Center, Denver, CO (Dr. Reims), University of British Columbia, BC, Canada (Dr. Davis), University College London, London, UK (Dr. Gainforth), and Stonybrook University School of Medicine, Stonybrook, NY (Dr. Cole [Emeritus]).
Abstract
- Objective: To describe Brief Action Planning (BAP), a structured, stepped-care self-management support technique for chronic illness care and disease prevention.
- Methods: A review of the theory and research supporting BAP and the questions and skills that comprise the technique with provision of a clinical example.
- Results: BAP facilitates goal setting and action planning to build self-efficacy for behavior change. It is grounded in the principles and practice of Motivational Interviewing and evidence-based constructs from the behavior change literature. Comprised of a series of 3 questions and 5 skills, BAP can be implemented by medical teams to help meet the self-management support objectives of the Patient-Centered Medical Home.
- Conclusion: BAP is a useful self-management support technique for busy medical practices to promote health behavior change and build patient self-efficacy for improved long-term clinical outcomes in chronic illness care and disease prevention.
Chronic disease is prevalent and time consuming, challenging, and expensive to manage [1]. Half of all adult primary care patients have more than 2 chronic diseases, and 75% of US health care dollars are spent on chronic illness care [2]. Given the health and financial impact of chronic disease, and recognizing that patients make daily decisions that affect disease control, efforts are needed to assist and empower patients to actively self-manage health behaviors that influence chronic illness outcomes. Patients who are supported to actively self-manage their own chronic illnesses have fewer symptoms, improved quality of life, and lower use of health care resources [3]. Historically, providers have tried to influence chronic illness self-management by advising behavior change (eg, smoking cessation, exercise) or telling patients to take medications; yet clinicians often become frustrated when patients do not “adhere” to their professional advice [4,5]. Many times, patients want to make changes that will improve their health but need support—commonly known as self-management support—to be successful.
Involving patients in decision making, emphasizing problem solving, setting goals, creating action plans (ie, when, where and how to enact a goal-directed behavior), and following up on goals are key features of successful self-management support methods [3,6–8]. Multiple approaches from the behavioral change literature, such as the 5 A’s (Assess, Advise, Agree, Assist, Arrange) [9], Motivational Interviewing (MI), and chronic disease self-management programs [10] have been used to provide more effective guidance for patients and their caregivers. However, the practicalities of these approaches in clinical settings have been questioned. The 5A’s, a counseling framework that is used to guide providers in health behavior change counseling, can feel overwhelming because it encompasses several different aspects of counseling [11,12]. Likewise, MI and adaptations of MI, which have been shown to outperform traditional “advice giving” in treatment of a broad range of behaviors and chronic conditions [13–16], have been critiqued since fidelity to this approach often involves multiple sessions of training, practice, and feedback to achieve proficiency [15,17,18]. Finally, while chronic disease self-management programs have been shown to be effective when used by peers in the community [10], similar results in primary care are not well established.
Given the challenges of providers practicing, learning, and using each of these approaches, efforts to develop an approach that supports patients to make behavioral changes that can be implemented in typical practice settings are needed. In addition, health delivery systems are transforming to team-based models with emphasis on leveraging each team member’s expertise and licensure [19]. In acknowledgement of these evolving practice realities, the National Committee for Quality Assurance (NCQA) included development and documentation of patient self-management plans and goals as a critical factor for achieving NCQA Patient-Centered Medical Home (PCMH) recognition [20]. Successful PCMH transformation therefore entails clinical practices developing effective and time efficient ways to incorporate self-management support strategies, a new service for many, into their care delivery systems often without additional staffing.
In this paper, we describe an evidence-informed, efficient self-management support technique called Brief Action Planning (BAP) [21–24]. BAP evolved into its current form through ongoing collaborative efforts of 4 of the authors (SC, DG, CD, KR) and is based on a foundation of original work by Steven Cole with contributions from Mary Cole in 2002 [25]. This technique addresses many of the barriers providers have cited to providing self-management support, as it can be used routinely by both individual providers and health care teams to facilitate patient-centered goal setting and action planning. BAP integrates principles and practice of MI with goal setting and action planning concepts from the self-management support, self-efficacy, and behavior change literature. In addition to reviewing the principles and theory that inform BAP, we introduce the steps of BAP and discuss practical considerations for incorporating BAP into clinical practice. In particular, we include suggestions about how BAP can be used in team-based clinical practice settings within the PCMH. Finally, we present a common clinical scenario to demonstrate BAP and provide resource links to online videos of BAP encounters. Throughout the paper, we use the word “clinician” to refer to professionals or other trained personnel using BAP, and “patient” to refer to those experiencing BAP, recognizing that other terms may be preferred in different settings.
What is BAP?
BAP is a highly structured, stepped-care, self-management support technique. Composed of a series of 3 questions and 5 skills (reviewed in detail below), BAP can be used to facilitate goal setting and action planning to build self-efficacy in chronic illness management and disease prevention [21–24]. The overall goal of BAP is to assist an individual to create an action plan for a self-management behavior that they feel confident that they can achieve. BAP is currently being used in diverse care settings including primary care, home health care, rehabilitation, mental health and public health to assist and empower patients to self-manage chronic illnesses and disabilities including diabetes, depression, spinal cord injury, arthritis, and hypertension. BAP is also being used to assist patients to develop action plans for disease prevention. For example, the Bellevue Hospital Personalized Prevention clinic, a pilot clinic that uses a mathematical model [26] to help patients and providers collaboratively prioritize prevention focus and strategies, systematically utilizes BAP as its self-management support technique for patient-centered action planning. At this time, BAP has been incorporated into teaching curriculums at multiple medical schools, presented at major national health care/academic conferences and is being increasingly integrated into health delivery systems across the United States and Canada to support patient self-management for NCQA-PCMH transformation. We have also developed a series of standardized programing to support fidelity in BAP skills development including a multidisciplinary introductory training curriculum, telephonic coaching, interactive web-based training tools, and a structured “Train the Trainer” curriculum [27]. In addition, a set of guidelines designed to ensure fidelity in BAP research has been developed [27].
Underlying Principles of BAP
BAP is grounded in the principles and practice of MI and the psychology of behavior change. Within behavior change, we draw primarily on self-efficacy and action planning theory and research. We discuss the key concepts in detail below.
The Spirit of MI
MI Spirit (Compassion, Acceptance, Partnership and Evocation) is an important overarching tenet for BAP. Compassionately supporting self-management with MI spirit involves a partnership with the patient rather than a prescription for change and the assurance that the clinician has the patients best interest always in mind (Compassion) [17]. Exemplifying “spirit” accepts that the ultimate choice to change is the patient’s alone (Acceptance) and acknowledges that individuals bring expertise about themselves and their lives to the conversation (Evocation). Adherence to “MI spirit” itself has been associated with positive behavior change outcomes in patients [5,28–32]. Demonstrating MI spirit throughout the change conversation is an essential foundational principle of BAP.
Action Planning and Self-Efficacy
In addition to the spirit of MI, BAP integrates 2 evidence-based constructs from the behavior change literature: action planning and self-efficacy [4,6,33–36]. Action planning requires that individuals specify when, where and how to enact a goal-directed behavior (eg, self-management behaviors). Action planning has been shown to mediate the intention-behavior relationship thereby increasing the likelihood that an individual’s intentions will lead to behavior change [37,38]. Given the demonstrated potential of action planning for ensuring individuals achieve their health goals, the BAP framework aspires to assist patients to create an action plan.
BAP also aims to build patients’ self-efficacy to enact the goals outlined in their action plans. Self-efficacy refers to a patient’s confidence in their ability to enact a behavior [33]. Several reviews of the literature have suggested a strong relationship between self-efficacy and adoption of healthy behaviors such as smoking cessation, weight control, contraception, alcohol abuse and physical activity [39–42]. Furthermore, Lorig et al demonstrated that the process of action planning itself contributes to enhanced self-efficacy [8]. BAP aims to build self-efficacy and ultimately change patients’ behaviors by helping patients to set an action plan that they feel confident in their ability to achieve.
Description of the BAP Steps
Three questions and 3 of the BAP skills (ie, SMART plan, eliciting a commitment statement, and follow-up) are applied during every BAP interaction, while 2 skills (ie, behavioral menu and problem solving for low confidence) are used as needed. The distinct functions and the evidence supporting the 3 questions and 5 BAP skills are described below.
Question 1: Eliciting a Behavioral Focus or Goal
Once engagement has been established and the clinician determines the patient is ready for self-management planning to occur, the first question of BAP can be asked: “Is there anything you would like to do for your health in the next week or two?”
Although technically Question 1 is a closed-ended question (in that it can be answered “yes” or “no”), in actual practice it generates productive discussions about change.
1) Have an Idea. A group of patients immediately present an idea that they are ready to do or are ready to consider doing. For these patients, clinicians can proceed directly to Skill 2—SMART Behavioral Planning; that is, asking patients directly if they are ready to turn their idea into a concrete plan. Some evidence suggests that further discussion, assessment, or even additional "motivational" exploration in patients who are ready to make a plan and already have an idea may actually decrease motivation for change [17, 32].
2) Not Sure. Another group of patients may want or need suggestions before committing to something specific they want to work on. For these patients, clinicians should use the opportunity to offer a Behavioral Menu (Skill 1).
3) No or Not at This Time. A third group of patients may not be interested or ready to make a change at this time or at all. Some in this group may be healthy or already self-managing effectively and have no need to make a plan, in which case the clinician acknowledges their active self-management and moves to the next part of the visit. Others in this group may have considerable ambivalence about change or face complex situations where other priorities take precedence. Clinicians frequently label these individuals as "resistant." The Spirit of MI can be very useful when working with these patients to accept and respect their autonomy while encouraging ongoing partnership at a future time. For example, a clinician may say “It sounds like you are not interested in making a plan for your health right now. Would it be OK if I ask you about this again at our next visit?” Pushing forward to make a "plan for change" when a patient is not ready decreases both motivation for change as well as the likelihood for a successful outcome [32].
Other patients may benefit from additional motivational approaches to further explore change and ambivalence. If the clinician does not have these skills, patients may be seamlessly transitioned to another resource within or external to the care team.
Skill 1: Offering a Behavioral Menu
If in response to Question 1 an individual is unable to come up with an idea of their own or needs more information, then offering a Behavioral Menu may be helpful [44,45]. Consistent with the “Spirit of MI,” BAP attempts to elicit ideas from the individual themselves; however, it is important to recognize that some people require assistance to identify possible actions. A behavioral menu is comprised of 2 or 3 suggestions or ideas that will ideally trigger individuals to discover an idea of their own. There are 3 distinct evidence-based steps to follow when presenting a Behavioral Menu.
1) Ask permission to offer a behavioral menu. Asking permission to share ideas respects patient autonomy and prevents the provider from inadvertently assuming an expert role. For example: “Would it be OK if I shared with you some examples of what some other patients I work with have done?”
2) Offer 2 to 3 general yet varied ideas all at once (Figure 2, entry 5). It helps to mention things that other patients have decided to do with some success. Using this approach avoids the clinician assuming too much about the patient or allowing the patient to reject the ideas. It is important to remember that the list is to prompt ideas, not to find a perfect solution [17]. For example: “One patient I work with decided to join a gym and start exercising, another decided to pick up an old hobby he used to enjoy doing and another patient decided to schedule some time with a friend she hadn’t seen in a while.”
3) Ask if any of the ideas appeal to the individual as something that might work for them or if the patient has an idea of his/her own (Figure 2, entry 5). Evocation from the Spirit of MI is built in with this prompt [17]. For example: “These are some ideas that have worked for other patients I work with, do they trigger any ideas that might work for you?”
Skill 2: SMART Planning
Once an individual decides on an area of focus, the clinician partners with the patient to clarify the details and create an action plan to achieve their goal. Given that individuals are more likely to successfully achieve goals that are specific, proximal, and achievable as opposed to vague and distal [46,47], the clinician works with patient to ensure that the patient’s goal is SMART (specific, measurable, achievable, relevant and time-bound). The term SMART has its roots in the business management literature [48] as an adaptation of Locke’s pioneering research (1968) on goal setting and motivation [49]. In particular, Locke and Latham’s theory of Goal Setting and Task performance, states that “specific and achievable” goals are more likely to be successfully reached [47,50].
We suggest helping the patient to make smart goals by eliciting answers to questions applicable to the plan, such as “what?” “where?” “when?” “how long?” “how often?” “how much?” and “when will you start?” [51]. A resulting plan might be “I will walk for 20 minutes, in my neighborhood, every Monday, Wednesday and Friday before dinner.”
Skill 3: Elicit a Commitment Statement
Once the individual has developed a specific plan, the next step of BAP is for the clinician to ask him or her to “tell back” the specifics of the plan. The provider might say something like, “Just to make sure we understand each other, would you repeat back what you’ve decided to do?” The act of “repeating back” organizes the details of the plan in the persons mind and may lead to an unconscious self-reflection about the feasibility of the plan [43,52], which then sets the stage for Question 2 of BAP (Scaling for Confidence). Commitment predicts subsequent behavior change, and the strength of the commitment language is the strongest predictor of success on an action plan [43,52,53]. For example saying “I will” is stronger than saying “I will try.”
Question 2: Scaling for Confidence
After a commitment statement has been elicited, the second question of BAP is asked. “How confident or sure do you feel about carrying out your plan on a scale from 0 to 10, where 0 is not confident at all and 10 is totally confident or sure?” Confidence scaling is a common tool used in behavioral interventions, MI, and chronic disease self-management programs [17,51]. Question 2 assesses an individual’s self-efficacy to complete the plan and facilitates discussion about potential barriers to implementation in order to increase the likelihood of success of a personal action plan.
For patients who have difficulty grasping the concept of a numerical scale, the word “sure” can be substituted for “confident” and a Likert scale including the terms “not at all sure,” “somewhat sure,” and “very sure” substituted for the numerical confidence ruler, ie, “How sure are you that you will be able to carry out your plan? Not at all sure, somewhat sure, or very sure?” Alternatively, people of different cultural backgrounds may find it easier to grasp the concept using familiar images or experiences. For example, Native Americans from the Southwest have adapted the scale to depict a series of images ranging from planting a corn seed to harvesting a crop or climbing a ladder, while in some Latino cultures the image of climbing a mountain (“How far up the mountain are you?”) is useful to demonstrate “level of confidence” concept [54].
Skill 4: Problem Solving for Low Confidence
When confidence is relatively low (ie, below 7), we suggest collaborative problem solving as the next step [8,51]. Low confidence or self-efficacy for plan completion is a concern since low self-efficacy predicts non-completion [8]. Successfully implementing the action plan, no matter how small, increases confidence and self-efficacy for engaging in the behavior [8].
There are several steps that a clinician follows when collaboratively problem-solving with a patient with low confidence (Figure 1).
• Recognize that a low confidence level is greater than no confidence at all. By affirming the strength of a patient’s confidence rather than negatively focusing on a low level of confidence, the provider emphasizes the patient’s strengths.
• Collaboratively explore ways that the plan could be modified in order to improve confidence. A Behavioral Menu can be offered if needed. For example, a clinician might say something like: “That’s great that your confidence level is a 5. A 5 is a lot higher than a 1. People are more likely to have success with their action plans when confidence levels are 7 or more. Do you have any ideas of how you might be able to increase your level confidence to a 7 or more?”
• If the patient has no ideas, ask permission to offer a Behavioral Menu: “Would it be ok to share some ideas about how other patients I’ve worked with have increased their confidence level?” If the patient agrees, then say... “Some people modify their plans to make them easier, some choose a less ambitious goal or adjust the frequency of their plan, and some people involve a friend or family member. Perhaps one of these ideas seems like a good one for you or maybe you have another idea?”
Question 3: Arranging Accountability
Once the details of the plan have been determined and confidence level for success is high, the next step is to ask Question 3: “Would you like to set a specific time to check in about your plan to see how things are going?” This question encourages a patient to be accountable for their plan, and reinforces the concept that the physician and care team consider the plan to be important. Research supports that people are more likely to follow through with a plan if they choose to report back their progress [43] and suggests that checking-in frequently earlier in the process is helpful [55]. Ideally the clinician and patient should agree on a time to check in on the plan within a week or two (Figure 2, entry 29).
Accountability in the form of a check-in may be arranged with the clinical provider, another member of the healthcare team or a support person of the patient’s choice (eg, spouse, friend). The patient may also choose to be accountable to themselves by using a calendar or a goal setting application on their smart phone device or computer.
Skill 5: Follow-up
The purpose of the check-in is for learning and adjustment of the plan as well as to provide support regardless of outcome. Checking-in encourages reflection on challenges and barriers as well as successes. Patients should be given guidance to think through what worked for them and what did not. Focusing just on “success” of the plan will be less helpful. If follow-up is not done with the care team in the near term, checking-in can be accomplished at the next scheduled visit. Patient portals provide another opportunity for patients to dialogue with the care team about their plan.
Experiential Insights from Clinical Experience Using BAP
The authors collective experience to date indicates that between 50% to 75% of individuals who are asked Question 1 go on to develop an action plan for change with relatively little need for additional skills. In other studies of action planning in primary care, 83% of patients made action plans during a visit, and at 3-week follow-up 53% had completed their action plan [56]. A recent study of action planning using an online self-management support program reported that action plans were successfully completed (49%), partially completed (40%) or incomplete (11% of the time) [35].
Another caveat to consider is that the process of planning is more important that the actual plan itself. It is imperative to allow the patient, not the clinician, to determine the plan. For example, a patient with multiple poorly controlled chronic illnesses including depression may decide to focus his action plan around cleaning out his car rather than disease control such as dietary modification, medication adherence or exercise. The clinician may initially fail to view this as a good use of clinician time or healthcare resources since it seems unrelated to health. However, successful completion of an action plan is not the only objective of action planning. Building self-efficacy, which may lead to additional action planning around health, is more important [4,46]. The challenge is therefore for the clinician to take a step back, relinquish the “expert role,” and support the goal setting process regardless of the plan. In this example, successfully cleaning out his car may increase the patient’s self-efficacy to control other aspects of his life including diet and the focus of future plans may shift [4].
When to Use BAP
Opportunities for patient engagement in action planning occur when addressing chronic illness concerns as well as during discussions about health maintenance and preventive care. BAP can be considered as part of any routine clinical agenda unless patient preferences or clinical acuity preclude it. As with most clinical encounters, the flow is often negotiated at the beginning of the visit. BAP can be accomplished at any time that works best for the flow and substance of the visit, but a few patterns have emerged based on our experience.
BAP fits naturally into the part of the visit when the care plan is being discussed. The term “care plan” is commonly used to describe all of the care that will be provided until the next visit. Care plans can include additional recommendations for testing or screening, therapeutic adjustments and or referrals for additional expertise. Ideally the patients “agreed upon” contribution to their care should also be captured and documented in their care plan. This is often described as the patients “self-management goal.” For patients who are ready to make a specific plan to change behavior, BAP is an efficient way to support patients to craft an action plan that can then be incorporated into the overall care plan.
Another variation of when to use BAP is the situation when the patient has had a prior action plan and is being seen for a recheck visit. Discussing the action plan early in the visit agenda focuses attention on the work patients have put into following their plan. Descriptions of success lead readily to action plans for the future. Time spent discussing failures or partial success is valuable to problem solve as well as to affirm continued efforts to self-manage.
BAP can also be used between scheduled visits. The check-in portion of BAP is particularly amenable to follow-up by phone or by another supporter. A pre-arranged follow-up 1 to 2 weeks after creation of a new action plan [8] provides encouragement to patients working on their plan and also helps identify those who need more support.
Finally, BAP can be completed over multiple visits. For patients who are thinking about change but are not yet committed to planning, a brief suggestion about the value of action planning with a behavioral menu may encourage additional self-reflection. Many times patients return to the next visit with clear ideas about changes that would be important for them to make.
Fitting BAP into a 20-Minute Visit
Using BAP is a time-efficient way to provide self-management support within the context of a 20-minute visit with engaged patients who are ready to set goals for health. With practice, clinicians can often conduct all the steps within 3 to 5 minutes. However, patients and clinicians often have competing demands and agendas and may not feel that they have time to conduct all the steps. Thus, utilizing other members of the health care team to deliver some or all of BAP can facilitate implementation.
Teams have been creative in their approach to BAP implementation but 2 common models involve a multidisciplinary approach to BAP. In one model, the clinician assesses the patient readiness to make a specific action plan by asking Question 1, usually after the current status of key problems have been addressed and discussions begin about the interim plan of care. If the patient indicates interest, another staff member trained in BAP, such as an medical assistant, health coach or nurse, guides the development of the specific plan, completes the remaining steps and inputs the patient’s BAP into the care plan.
In another commonly deployed model, the front desk clerk or medical assistant helps to get the patient thinking by asking Question 1 and perhaps by providing a behavioral menu. When the clinician sees the patient, he follows up on the behavior change the patient has chosen and affirms the choice. Clinicians often flex seamlessly with other team members to complete the action plan depending on the schedule and current patient flow.
Regardless of how the workflows are designed, BAP implementation requires staff that can provide BAP with fidelity, effective communication among team members involved in the process and a standardized approach to documentation of the specific action plan, plan for check-in and notes about follow-up. Care teams commonly test different variations of personnel and workflows to find what works best for their particular practice.
Implementing BAP to Support PCMH Transformation
To support PCMH transformation substantial changes are needed to make care more proactive, more patient-centered and more accountable. One of the common elements for PCMH recognition regardless of sponsor is to enhance self-management support [20,57,58]. Practices pursuing PCMH designation are searching for effective evidence-based approaches to provide self-management support and guide action planning for patients. The authors suggest implementation of BAP as a potential strategy to enhance self-management support. In addition to facilitating meeting the actual PCMH criteria, BAP is aligned with the transitions in care delivery that are an important part of the transformation including reliance on team-based care and meaningful engagement of patients in their care [59,60].
In our experience, BAP is introduced incrementally into a practice initially focusing on one or two patient segments and then including more as resources allow. Successful BAP implementation begins with an organizational commitment to self-management support, decisions about which populations would benefit most from self-management support and BAP, training of key staff and clearly defined workflows that ensure reliable BAP provision.
BAP’s stepped-care design makes it easy to teach to all team members and as described above, team-based delivery of BAP functions well in those situations where clinicians and trained ancillary staff can “hand off” the process at any time to optimize the value to the patient while respecting inherent time constraints.
Documentation of the actual goal and follow-up is an important component to fully leverage BAP. Goals captured in a template generate actionable lists for action plan follow-up. Since EHRs vary considerably in their capacity to capture goals, teams adding BAP to their workflow will benefit from discussion of standardized documentation practices and forms.
Summary
Brief Action Planning is a self-management support technique that can be used in busy clinical settings to support patient self-management through patient-centered goal setting. Each step of BAP is based on principles grounded in evidence. Health care teams can learn BAP and integrate it into clinical delivery systems to support self-management for PCMH transformation.
Corresponding author: Damara Gutnick, MD, New York University School of Medicine, New York, NY, damaragutnick@gmail.com.
Financial disclosures: None.
From the New York University School of Medicine, New York, NY (Drs. Gutnick and Jay), University of Colorado Health Sciences Center, Denver, CO (Dr. Reims), University of British Columbia, BC, Canada (Dr. Davis), University College London, London, UK (Dr. Gainforth), and Stonybrook University School of Medicine, Stonybrook, NY (Dr. Cole [Emeritus]).
Abstract
- Objective: To describe Brief Action Planning (BAP), a structured, stepped-care self-management support technique for chronic illness care and disease prevention.
- Methods: A review of the theory and research supporting BAP and the questions and skills that comprise the technique with provision of a clinical example.
- Results: BAP facilitates goal setting and action planning to build self-efficacy for behavior change. It is grounded in the principles and practice of Motivational Interviewing and evidence-based constructs from the behavior change literature. Comprised of a series of 3 questions and 5 skills, BAP can be implemented by medical teams to help meet the self-management support objectives of the Patient-Centered Medical Home.
- Conclusion: BAP is a useful self-management support technique for busy medical practices to promote health behavior change and build patient self-efficacy for improved long-term clinical outcomes in chronic illness care and disease prevention.
Chronic disease is prevalent and time consuming, challenging, and expensive to manage [1]. Half of all adult primary care patients have more than 2 chronic diseases, and 75% of US health care dollars are spent on chronic illness care [2]. Given the health and financial impact of chronic disease, and recognizing that patients make daily decisions that affect disease control, efforts are needed to assist and empower patients to actively self-manage health behaviors that influence chronic illness outcomes. Patients who are supported to actively self-manage their own chronic illnesses have fewer symptoms, improved quality of life, and lower use of health care resources [3]. Historically, providers have tried to influence chronic illness self-management by advising behavior change (eg, smoking cessation, exercise) or telling patients to take medications; yet clinicians often become frustrated when patients do not “adhere” to their professional advice [4,5]. Many times, patients want to make changes that will improve their health but need support—commonly known as self-management support—to be successful.
Involving patients in decision making, emphasizing problem solving, setting goals, creating action plans (ie, when, where and how to enact a goal-directed behavior), and following up on goals are key features of successful self-management support methods [3,6–8]. Multiple approaches from the behavioral change literature, such as the 5 A’s (Assess, Advise, Agree, Assist, Arrange) [9], Motivational Interviewing (MI), and chronic disease self-management programs [10] have been used to provide more effective guidance for patients and their caregivers. However, the practicalities of these approaches in clinical settings have been questioned. The 5A’s, a counseling framework that is used to guide providers in health behavior change counseling, can feel overwhelming because it encompasses several different aspects of counseling [11,12]. Likewise, MI and adaptations of MI, which have been shown to outperform traditional “advice giving” in treatment of a broad range of behaviors and chronic conditions [13–16], have been critiqued since fidelity to this approach often involves multiple sessions of training, practice, and feedback to achieve proficiency [15,17,18]. Finally, while chronic disease self-management programs have been shown to be effective when used by peers in the community [10], similar results in primary care are not well established.
Given the challenges of providers practicing, learning, and using each of these approaches, efforts to develop an approach that supports patients to make behavioral changes that can be implemented in typical practice settings are needed. In addition, health delivery systems are transforming to team-based models with emphasis on leveraging each team member’s expertise and licensure [19]. In acknowledgement of these evolving practice realities, the National Committee for Quality Assurance (NCQA) included development and documentation of patient self-management plans and goals as a critical factor for achieving NCQA Patient-Centered Medical Home (PCMH) recognition [20]. Successful PCMH transformation therefore entails clinical practices developing effective and time efficient ways to incorporate self-management support strategies, a new service for many, into their care delivery systems often without additional staffing.
In this paper, we describe an evidence-informed, efficient self-management support technique called Brief Action Planning (BAP) [21–24]. BAP evolved into its current form through ongoing collaborative efforts of 4 of the authors (SC, DG, CD, KR) and is based on a foundation of original work by Steven Cole with contributions from Mary Cole in 2002 [25]. This technique addresses many of the barriers providers have cited to providing self-management support, as it can be used routinely by both individual providers and health care teams to facilitate patient-centered goal setting and action planning. BAP integrates principles and practice of MI with goal setting and action planning concepts from the self-management support, self-efficacy, and behavior change literature. In addition to reviewing the principles and theory that inform BAP, we introduce the steps of BAP and discuss practical considerations for incorporating BAP into clinical practice. In particular, we include suggestions about how BAP can be used in team-based clinical practice settings within the PCMH. Finally, we present a common clinical scenario to demonstrate BAP and provide resource links to online videos of BAP encounters. Throughout the paper, we use the word “clinician” to refer to professionals or other trained personnel using BAP, and “patient” to refer to those experiencing BAP, recognizing that other terms may be preferred in different settings.
What is BAP?
BAP is a highly structured, stepped-care, self-management support technique. Composed of a series of 3 questions and 5 skills (reviewed in detail below), BAP can be used to facilitate goal setting and action planning to build self-efficacy in chronic illness management and disease prevention [21–24]. The overall goal of BAP is to assist an individual to create an action plan for a self-management behavior that they feel confident that they can achieve. BAP is currently being used in diverse care settings including primary care, home health care, rehabilitation, mental health and public health to assist and empower patients to self-manage chronic illnesses and disabilities including diabetes, depression, spinal cord injury, arthritis, and hypertension. BAP is also being used to assist patients to develop action plans for disease prevention. For example, the Bellevue Hospital Personalized Prevention clinic, a pilot clinic that uses a mathematical model [26] to help patients and providers collaboratively prioritize prevention focus and strategies, systematically utilizes BAP as its self-management support technique for patient-centered action planning. At this time, BAP has been incorporated into teaching curriculums at multiple medical schools, presented at major national health care/academic conferences and is being increasingly integrated into health delivery systems across the United States and Canada to support patient self-management for NCQA-PCMH transformation. We have also developed a series of standardized programing to support fidelity in BAP skills development including a multidisciplinary introductory training curriculum, telephonic coaching, interactive web-based training tools, and a structured “Train the Trainer” curriculum [27]. In addition, a set of guidelines designed to ensure fidelity in BAP research has been developed [27].
Underlying Principles of BAP
BAP is grounded in the principles and practice of MI and the psychology of behavior change. Within behavior change, we draw primarily on self-efficacy and action planning theory and research. We discuss the key concepts in detail below.
The Spirit of MI
MI Spirit (Compassion, Acceptance, Partnership and Evocation) is an important overarching tenet for BAP. Compassionately supporting self-management with MI spirit involves a partnership with the patient rather than a prescription for change and the assurance that the clinician has the patients best interest always in mind (Compassion) [17]. Exemplifying “spirit” accepts that the ultimate choice to change is the patient’s alone (Acceptance) and acknowledges that individuals bring expertise about themselves and their lives to the conversation (Evocation). Adherence to “MI spirit” itself has been associated with positive behavior change outcomes in patients [5,28–32]. Demonstrating MI spirit throughout the change conversation is an essential foundational principle of BAP.
Action Planning and Self-Efficacy
In addition to the spirit of MI, BAP integrates 2 evidence-based constructs from the behavior change literature: action planning and self-efficacy [4,6,33–36]. Action planning requires that individuals specify when, where and how to enact a goal-directed behavior (eg, self-management behaviors). Action planning has been shown to mediate the intention-behavior relationship thereby increasing the likelihood that an individual’s intentions will lead to behavior change [37,38]. Given the demonstrated potential of action planning for ensuring individuals achieve their health goals, the BAP framework aspires to assist patients to create an action plan.
BAP also aims to build patients’ self-efficacy to enact the goals outlined in their action plans. Self-efficacy refers to a patient’s confidence in their ability to enact a behavior [33]. Several reviews of the literature have suggested a strong relationship between self-efficacy and adoption of healthy behaviors such as smoking cessation, weight control, contraception, alcohol abuse and physical activity [39–42]. Furthermore, Lorig et al demonstrated that the process of action planning itself contributes to enhanced self-efficacy [8]. BAP aims to build self-efficacy and ultimately change patients’ behaviors by helping patients to set an action plan that they feel confident in their ability to achieve.
Description of the BAP Steps
Three questions and 3 of the BAP skills (ie, SMART plan, eliciting a commitment statement, and follow-up) are applied during every BAP interaction, while 2 skills (ie, behavioral menu and problem solving for low confidence) are used as needed. The distinct functions and the evidence supporting the 3 questions and 5 BAP skills are described below.
Question 1: Eliciting a Behavioral Focus or Goal
Once engagement has been established and the clinician determines the patient is ready for self-management planning to occur, the first question of BAP can be asked: “Is there anything you would like to do for your health in the next week or two?”
Although technically Question 1 is a closed-ended question (in that it can be answered “yes” or “no”), in actual practice it generates productive discussions about change.
1) Have an Idea. A group of patients immediately present an idea that they are ready to do or are ready to consider doing. For these patients, clinicians can proceed directly to Skill 2—SMART Behavioral Planning; that is, asking patients directly if they are ready to turn their idea into a concrete plan. Some evidence suggests that further discussion, assessment, or even additional "motivational" exploration in patients who are ready to make a plan and already have an idea may actually decrease motivation for change [17, 32].
2) Not Sure. Another group of patients may want or need suggestions before committing to something specific they want to work on. For these patients, clinicians should use the opportunity to offer a Behavioral Menu (Skill 1).
3) No or Not at This Time. A third group of patients may not be interested or ready to make a change at this time or at all. Some in this group may be healthy or already self-managing effectively and have no need to make a plan, in which case the clinician acknowledges their active self-management and moves to the next part of the visit. Others in this group may have considerable ambivalence about change or face complex situations where other priorities take precedence. Clinicians frequently label these individuals as "resistant." The Spirit of MI can be very useful when working with these patients to accept and respect their autonomy while encouraging ongoing partnership at a future time. For example, a clinician may say “It sounds like you are not interested in making a plan for your health right now. Would it be OK if I ask you about this again at our next visit?” Pushing forward to make a "plan for change" when a patient is not ready decreases both motivation for change as well as the likelihood for a successful outcome [32].
Other patients may benefit from additional motivational approaches to further explore change and ambivalence. If the clinician does not have these skills, patients may be seamlessly transitioned to another resource within or external to the care team.
Skill 1: Offering a Behavioral Menu
If in response to Question 1 an individual is unable to come up with an idea of their own or needs more information, then offering a Behavioral Menu may be helpful [44,45]. Consistent with the “Spirit of MI,” BAP attempts to elicit ideas from the individual themselves; however, it is important to recognize that some people require assistance to identify possible actions. A behavioral menu is comprised of 2 or 3 suggestions or ideas that will ideally trigger individuals to discover an idea of their own. There are 3 distinct evidence-based steps to follow when presenting a Behavioral Menu.
1) Ask permission to offer a behavioral menu. Asking permission to share ideas respects patient autonomy and prevents the provider from inadvertently assuming an expert role. For example: “Would it be OK if I shared with you some examples of what some other patients I work with have done?”
2) Offer 2 to 3 general yet varied ideas all at once (Figure 2, entry 5). It helps to mention things that other patients have decided to do with some success. Using this approach avoids the clinician assuming too much about the patient or allowing the patient to reject the ideas. It is important to remember that the list is to prompt ideas, not to find a perfect solution [17]. For example: “One patient I work with decided to join a gym and start exercising, another decided to pick up an old hobby he used to enjoy doing and another patient decided to schedule some time with a friend she hadn’t seen in a while.”
3) Ask if any of the ideas appeal to the individual as something that might work for them or if the patient has an idea of his/her own (Figure 2, entry 5). Evocation from the Spirit of MI is built in with this prompt [17]. For example: “These are some ideas that have worked for other patients I work with, do they trigger any ideas that might work for you?”
Skill 2: SMART Planning
Once an individual decides on an area of focus, the clinician partners with the patient to clarify the details and create an action plan to achieve their goal. Given that individuals are more likely to successfully achieve goals that are specific, proximal, and achievable as opposed to vague and distal [46,47], the clinician works with patient to ensure that the patient’s goal is SMART (specific, measurable, achievable, relevant and time-bound). The term SMART has its roots in the business management literature [48] as an adaptation of Locke’s pioneering research (1968) on goal setting and motivation [49]. In particular, Locke and Latham’s theory of Goal Setting and Task performance, states that “specific and achievable” goals are more likely to be successfully reached [47,50].
We suggest helping the patient to make smart goals by eliciting answers to questions applicable to the plan, such as “what?” “where?” “when?” “how long?” “how often?” “how much?” and “when will you start?” [51]. A resulting plan might be “I will walk for 20 minutes, in my neighborhood, every Monday, Wednesday and Friday before dinner.”
Skill 3: Elicit a Commitment Statement
Once the individual has developed a specific plan, the next step of BAP is for the clinician to ask him or her to “tell back” the specifics of the plan. The provider might say something like, “Just to make sure we understand each other, would you repeat back what you’ve decided to do?” The act of “repeating back” organizes the details of the plan in the persons mind and may lead to an unconscious self-reflection about the feasibility of the plan [43,52], which then sets the stage for Question 2 of BAP (Scaling for Confidence). Commitment predicts subsequent behavior change, and the strength of the commitment language is the strongest predictor of success on an action plan [43,52,53]. For example saying “I will” is stronger than saying “I will try.”
Question 2: Scaling for Confidence
After a commitment statement has been elicited, the second question of BAP is asked. “How confident or sure do you feel about carrying out your plan on a scale from 0 to 10, where 0 is not confident at all and 10 is totally confident or sure?” Confidence scaling is a common tool used in behavioral interventions, MI, and chronic disease self-management programs [17,51]. Question 2 assesses an individual’s self-efficacy to complete the plan and facilitates discussion about potential barriers to implementation in order to increase the likelihood of success of a personal action plan.
For patients who have difficulty grasping the concept of a numerical scale, the word “sure” can be substituted for “confident” and a Likert scale including the terms “not at all sure,” “somewhat sure,” and “very sure” substituted for the numerical confidence ruler, ie, “How sure are you that you will be able to carry out your plan? Not at all sure, somewhat sure, or very sure?” Alternatively, people of different cultural backgrounds may find it easier to grasp the concept using familiar images or experiences. For example, Native Americans from the Southwest have adapted the scale to depict a series of images ranging from planting a corn seed to harvesting a crop or climbing a ladder, while in some Latino cultures the image of climbing a mountain (“How far up the mountain are you?”) is useful to demonstrate “level of confidence” concept [54].
Skill 4: Problem Solving for Low Confidence
When confidence is relatively low (ie, below 7), we suggest collaborative problem solving as the next step [8,51]. Low confidence or self-efficacy for plan completion is a concern since low self-efficacy predicts non-completion [8]. Successfully implementing the action plan, no matter how small, increases confidence and self-efficacy for engaging in the behavior [8].
There are several steps that a clinician follows when collaboratively problem-solving with a patient with low confidence (Figure 1).
• Recognize that a low confidence level is greater than no confidence at all. By affirming the strength of a patient’s confidence rather than negatively focusing on a low level of confidence, the provider emphasizes the patient’s strengths.
• Collaboratively explore ways that the plan could be modified in order to improve confidence. A Behavioral Menu can be offered if needed. For example, a clinician might say something like: “That’s great that your confidence level is a 5. A 5 is a lot higher than a 1. People are more likely to have success with their action plans when confidence levels are 7 or more. Do you have any ideas of how you might be able to increase your level confidence to a 7 or more?”
• If the patient has no ideas, ask permission to offer a Behavioral Menu: “Would it be ok to share some ideas about how other patients I’ve worked with have increased their confidence level?” If the patient agrees, then say... “Some people modify their plans to make them easier, some choose a less ambitious goal or adjust the frequency of their plan, and some people involve a friend or family member. Perhaps one of these ideas seems like a good one for you or maybe you have another idea?”
Question 3: Arranging Accountability
Once the details of the plan have been determined and confidence level for success is high, the next step is to ask Question 3: “Would you like to set a specific time to check in about your plan to see how things are going?” This question encourages a patient to be accountable for their plan, and reinforces the concept that the physician and care team consider the plan to be important. Research supports that people are more likely to follow through with a plan if they choose to report back their progress [43] and suggests that checking-in frequently earlier in the process is helpful [55]. Ideally the clinician and patient should agree on a time to check in on the plan within a week or two (Figure 2, entry 29).
Accountability in the form of a check-in may be arranged with the clinical provider, another member of the healthcare team or a support person of the patient’s choice (eg, spouse, friend). The patient may also choose to be accountable to themselves by using a calendar or a goal setting application on their smart phone device or computer.
Skill 5: Follow-up
The purpose of the check-in is for learning and adjustment of the plan as well as to provide support regardless of outcome. Checking-in encourages reflection on challenges and barriers as well as successes. Patients should be given guidance to think through what worked for them and what did not. Focusing just on “success” of the plan will be less helpful. If follow-up is not done with the care team in the near term, checking-in can be accomplished at the next scheduled visit. Patient portals provide another opportunity for patients to dialogue with the care team about their plan.
Experiential Insights from Clinical Experience Using BAP
The authors collective experience to date indicates that between 50% to 75% of individuals who are asked Question 1 go on to develop an action plan for change with relatively little need for additional skills. In other studies of action planning in primary care, 83% of patients made action plans during a visit, and at 3-week follow-up 53% had completed their action plan [56]. A recent study of action planning using an online self-management support program reported that action plans were successfully completed (49%), partially completed (40%) or incomplete (11% of the time) [35].
Another caveat to consider is that the process of planning is more important that the actual plan itself. It is imperative to allow the patient, not the clinician, to determine the plan. For example, a patient with multiple poorly controlled chronic illnesses including depression may decide to focus his action plan around cleaning out his car rather than disease control such as dietary modification, medication adherence or exercise. The clinician may initially fail to view this as a good use of clinician time or healthcare resources since it seems unrelated to health. However, successful completion of an action plan is not the only objective of action planning. Building self-efficacy, which may lead to additional action planning around health, is more important [4,46]. The challenge is therefore for the clinician to take a step back, relinquish the “expert role,” and support the goal setting process regardless of the plan. In this example, successfully cleaning out his car may increase the patient’s self-efficacy to control other aspects of his life including diet and the focus of future plans may shift [4].
When to Use BAP
Opportunities for patient engagement in action planning occur when addressing chronic illness concerns as well as during discussions about health maintenance and preventive care. BAP can be considered as part of any routine clinical agenda unless patient preferences or clinical acuity preclude it. As with most clinical encounters, the flow is often negotiated at the beginning of the visit. BAP can be accomplished at any time that works best for the flow and substance of the visit, but a few patterns have emerged based on our experience.
BAP fits naturally into the part of the visit when the care plan is being discussed. The term “care plan” is commonly used to describe all of the care that will be provided until the next visit. Care plans can include additional recommendations for testing or screening, therapeutic adjustments and or referrals for additional expertise. Ideally the patients “agreed upon” contribution to their care should also be captured and documented in their care plan. This is often described as the patients “self-management goal.” For patients who are ready to make a specific plan to change behavior, BAP is an efficient way to support patients to craft an action plan that can then be incorporated into the overall care plan.
Another variation of when to use BAP is the situation when the patient has had a prior action plan and is being seen for a recheck visit. Discussing the action plan early in the visit agenda focuses attention on the work patients have put into following their plan. Descriptions of success lead readily to action plans for the future. Time spent discussing failures or partial success is valuable to problem solve as well as to affirm continued efforts to self-manage.
BAP can also be used between scheduled visits. The check-in portion of BAP is particularly amenable to follow-up by phone or by another supporter. A pre-arranged follow-up 1 to 2 weeks after creation of a new action plan [8] provides encouragement to patients working on their plan and also helps identify those who need more support.
Finally, BAP can be completed over multiple visits. For patients who are thinking about change but are not yet committed to planning, a brief suggestion about the value of action planning with a behavioral menu may encourage additional self-reflection. Many times patients return to the next visit with clear ideas about changes that would be important for them to make.
Fitting BAP into a 20-Minute Visit
Using BAP is a time-efficient way to provide self-management support within the context of a 20-minute visit with engaged patients who are ready to set goals for health. With practice, clinicians can often conduct all the steps within 3 to 5 minutes. However, patients and clinicians often have competing demands and agendas and may not feel that they have time to conduct all the steps. Thus, utilizing other members of the health care team to deliver some or all of BAP can facilitate implementation.
Teams have been creative in their approach to BAP implementation but 2 common models involve a multidisciplinary approach to BAP. In one model, the clinician assesses the patient readiness to make a specific action plan by asking Question 1, usually after the current status of key problems have been addressed and discussions begin about the interim plan of care. If the patient indicates interest, another staff member trained in BAP, such as an medical assistant, health coach or nurse, guides the development of the specific plan, completes the remaining steps and inputs the patient’s BAP into the care plan.
In another commonly deployed model, the front desk clerk or medical assistant helps to get the patient thinking by asking Question 1 and perhaps by providing a behavioral menu. When the clinician sees the patient, he follows up on the behavior change the patient has chosen and affirms the choice. Clinicians often flex seamlessly with other team members to complete the action plan depending on the schedule and current patient flow.
Regardless of how the workflows are designed, BAP implementation requires staff that can provide BAP with fidelity, effective communication among team members involved in the process and a standardized approach to documentation of the specific action plan, plan for check-in and notes about follow-up. Care teams commonly test different variations of personnel and workflows to find what works best for their particular practice.
Implementing BAP to Support PCMH Transformation
To support PCMH transformation substantial changes are needed to make care more proactive, more patient-centered and more accountable. One of the common elements for PCMH recognition regardless of sponsor is to enhance self-management support [20,57,58]. Practices pursuing PCMH designation are searching for effective evidence-based approaches to provide self-management support and guide action planning for patients. The authors suggest implementation of BAP as a potential strategy to enhance self-management support. In addition to facilitating meeting the actual PCMH criteria, BAP is aligned with the transitions in care delivery that are an important part of the transformation including reliance on team-based care and meaningful engagement of patients in their care [59,60].
In our experience, BAP is introduced incrementally into a practice initially focusing on one or two patient segments and then including more as resources allow. Successful BAP implementation begins with an organizational commitment to self-management support, decisions about which populations would benefit most from self-management support and BAP, training of key staff and clearly defined workflows that ensure reliable BAP provision.
BAP’s stepped-care design makes it easy to teach to all team members and as described above, team-based delivery of BAP functions well in those situations where clinicians and trained ancillary staff can “hand off” the process at any time to optimize the value to the patient while respecting inherent time constraints.
Documentation of the actual goal and follow-up is an important component to fully leverage BAP. Goals captured in a template generate actionable lists for action plan follow-up. Since EHRs vary considerably in their capacity to capture goals, teams adding BAP to their workflow will benefit from discussion of standardized documentation practices and forms.
Summary
Brief Action Planning is a self-management support technique that can be used in busy clinical settings to support patient self-management through patient-centered goal setting. Each step of BAP is based on principles grounded in evidence. Health care teams can learn BAP and integrate it into clinical delivery systems to support self-management for PCMH transformation.
Corresponding author: Damara Gutnick, MD, New York University School of Medicine, New York, NY, damaragutnick@gmail.com.
Financial disclosures: None.
1. Hoffman C, Rice D, Sung HY. Persons withnic conditions. Their prevalence and costs. JAMA 1996;276(18):1473–9.
2. Institute of Medicine. Living well with chro:ic illness: a call for public health action. Washington (DC); The National Academies Press; 2012.
3. De Silva D. Evidence: helping people help themselves. London: The Health Foundation Inspiring Improvement; 2011.
4. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–75.
5. Miller W, Benefield R, Tonigan J. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. J Consul Clin Psychol 1993;61:455–461.
6. Lorig K, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7.
7. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation 2010;122:406–41.
8. Lorig K, Laurent DD, Plant K, Krishnan E, Ritter PL. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013. Available at www.ncbi.nlm.nih.gov/pubmed/23838837.
9. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high-quality obesity counseling in primary care using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
10. Lorig KR, Ritter P, Stewart a L, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
11. Jay MR, Gillespie CC, Schlair SL, et al. The impact of primary care resident physician training on patient weight loss at 12 months. Obesity 2013;21:45–50.
12. Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med 2004;27:61–79.
13. Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93:157–68.
14. Rubak S, Sandbæk A, Lauritzen T, Christensen B. Motivational Interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–12.
15. Dunn C, Deroo L, Rivara F. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725–42.
16. Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control 2010;19:410–6.
17. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York: Guilford Press; 2013.
18. Resnicow K, DiIorio C, Soet J, et al. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol 2002;21:444–451.
19. Doherty RB, Crowley RA. Principles supporting dynamic clinical care teams: an American College of Physicians position paper. Ann Intern Med 2013;159:620–6.
20. NCQA PCMH 2011 Standards, Elements and Factors. Documentation Guideline/Data Sources. 4A: Provide self-care support and community resources. Available at www.ncqa.org/portals/0/Programs/Recognition/PCMH_2011_Data_Sources_6.6.12.pdf.
21. Reims K, Gutnick D, Davis C, Cole S. Brief action planning white paper. 2012. Available at www.centrecmi.ca.
22. Cole S, Davis C, Cole M, Gutnick D. Motivational interviewing and the patient centered medical home: a strategic approach to self-management support in primary care. In: Patient-Centered Primary Care Collaborative. Health IT in the patient centered medical home. October 2010. Available at www.pcpcc.net/guide/health-it-pcmh.
23. Cole S, Cole M, Gutnick D, Davis C. Function three: collaborate for management. In: Cole S, Bird J, editors. The medical interview: the three function approach. 3rd ed. Philadelphia:Saunders; 2014.
24. Cole S, Gutnick D, Davis C, Cole M. Brief action planning (BAP): a self-management support tool. In: Bickley L. Bates’ guide to physical examination and history taking. 11th ed. Philadelphia: Lippincott Williams and Wilkins; 2013.
25. AMA Physician tip sheet for self-management support. Available at www.ama-assn.org/ama1/pub/upload/mm/433/phys_tip_sheet.pdf.
26. Taksler G, Keshner M, Fagerlin A. Personalized estimates of benefit from preventive care guidelines. Ann Intern Med 2013;159:161–9.
27. Centre for Comprehensive Motivational Interventions [website]. Available at www.centreecmi.com.
28. Del Canale S, Louis DZ, Maio V, et al. The relationship between physician empathy and disease complications: an empirical study of primary care physicians and their diabetic patients in Parma, Italy. Acad Med 2012;87:1243–9.
29. Moyers TB, Miller WR, Hendrickson SML. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. J Consult Clin Psychol 2005;73:590–8.
30. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
31. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
32. Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37:129–40.
33. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;85:191–215.
34. Kiesler, Charles A. The psychology of commitment: experiments linking behavior to belief. New York: Academic Press;1971.
35. Lorig K, Laurent DD, Plant K, et al. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013.
36. MacGregor K, Handley M, Wong S, et al. Behavior-change action plans in primary care: a feasibility study of clinicians. J Am Board Fam Med 19:215–23.
37. Gollwitzer P. Implementation intentions. Am Psychol 1999;54:493–503.
38. Gollwitzer P, Sheeran P. Implementation intensions and goal achievement: A meta-analysis of effects and processes. Adv Exp Soc Psychology 2006;38:69–119.
39. Stretcher V, De Vellis B, Becker M, Rosenstock I. The role of self-efficacy in achieving behavior change. Health Educ Q 1986;13:73–92.
40. Ajzen I. Constructing a theory of planned behavior questionnaire. Available at people.umass.edu/aizen/pdf/tpb.measurement.pdf.
41. Rogers RW. Protection motivation theory of fear appeals and attitude-change. J Psychol 1975;91:93–114.
42. Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol An Int Rev 2008;57:1–29.
43. Cialdini R. Influence: science and practice. 5th ed. Boston:Allyn and Bacon; 2008.
44. Stott NC, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: diabetes care and negotiating skills. Fam Pract 1995;12:413–8.
45. Miller WR, Rollnick S, Butler C. Motivational interviewing in health care. New York: Guilford Press; 2008.
46. Bodenheimer T, Handley M. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns 2009;76:174–80.
47. Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. Am Psychol 2002;57:705–17.
48. Doran G. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev 1981;70:35–6.
49. Locke EA. Toward a theory of task motivation and incentives. Organ Behav Hum Perform 1968;3:157–89.
50. Locke EA, Latham GP, Erez M. The determinants of goal commitment. Acad Manag Rev 1988;13:23–39.
51. Lorig K, Homan H, Sobel D, et al. Living a healthy life with chronic conditions. 4th ed. Boulder: Bull Publishing; 2012.
52. Amrhein PC, Miller WR, Yahne CE, et al. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862–78.
53. Ahaeonovich E, Amrhein PC, Bisaha A, et al. Cognition, commitment language and behavioral change among cocaine-dependent patients. Psychol Addict Behav 2008;22:557–62.
54. Gutnick D. Centre for Comprehensive Motivational Interventions community of practice webinar. Brief action planning and culture: developing culturally specific confidence rules. 2012. Available at www.centrecmi.ca.
55. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults. A scientific statement from the American Heart Association. Circulation 2010;122:406–41.
56. Handley M, MacGregor K, Schillinger D, et al. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med 2006;19:224–31.
57. Joint Commision. Primary care medical home option-additional requirements. Available at www.jointcommission.org/assets/1/18/PCMH_new_stds_by_5_characteristics.pdf.
58. Oregon Health Policy and Research. Standards for patient centered medical home recognition. Available at www.oregon.gov/oha/OHPR/pages/healthreform/pcpch/standards.aspx.
59. Nutting PA, Crabtree BF, Miller WL, et al. Journey to the patient-centered medical home: a qualitative analysis of the experiences of practices in the national demonstration project. Am Fam Med 2010;8(Suppl 1):S45–S56.
60. Stewart EE, Nutting PA, Crabtree BF, et al. Implementing the patient-centered medical home: observation and description of the National Demonstration Project. Am Fam Med 2010;8(Suppl 1):S21–S32.
1. Hoffman C, Rice D, Sung HY. Persons withnic conditions. Their prevalence and costs. JAMA 1996;276(18):1473–9.
2. Institute of Medicine. Living well with chro:ic illness: a call for public health action. Washington (DC); The National Academies Press; 2012.
3. De Silva D. Evidence: helping people help themselves. London: The Health Foundation Inspiring Improvement; 2011.
4. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–75.
5. Miller W, Benefield R, Tonigan J. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. J Consul Clin Psychol 1993;61:455–461.
6. Lorig K, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7.
7. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation 2010;122:406–41.
8. Lorig K, Laurent DD, Plant K, Krishnan E, Ritter PL. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013. Available at www.ncbi.nlm.nih.gov/pubmed/23838837.
9. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high-quality obesity counseling in primary care using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
10. Lorig KR, Ritter P, Stewart a L, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
11. Jay MR, Gillespie CC, Schlair SL, et al. The impact of primary care resident physician training on patient weight loss at 12 months. Obesity 2013;21:45–50.
12. Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med 2004;27:61–79.
13. Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93:157–68.
14. Rubak S, Sandbæk A, Lauritzen T, Christensen B. Motivational Interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–12.
15. Dunn C, Deroo L, Rivara F. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725–42.
16. Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control 2010;19:410–6.
17. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York: Guilford Press; 2013.
18. Resnicow K, DiIorio C, Soet J, et al. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol 2002;21:444–451.
19. Doherty RB, Crowley RA. Principles supporting dynamic clinical care teams: an American College of Physicians position paper. Ann Intern Med 2013;159:620–6.
20. NCQA PCMH 2011 Standards, Elements and Factors. Documentation Guideline/Data Sources. 4A: Provide self-care support and community resources. Available at www.ncqa.org/portals/0/Programs/Recognition/PCMH_2011_Data_Sources_6.6.12.pdf.
21. Reims K, Gutnick D, Davis C, Cole S. Brief action planning white paper. 2012. Available at www.centrecmi.ca.
22. Cole S, Davis C, Cole M, Gutnick D. Motivational interviewing and the patient centered medical home: a strategic approach to self-management support in primary care. In: Patient-Centered Primary Care Collaborative. Health IT in the patient centered medical home. October 2010. Available at www.pcpcc.net/guide/health-it-pcmh.
23. Cole S, Cole M, Gutnick D, Davis C. Function three: collaborate for management. In: Cole S, Bird J, editors. The medical interview: the three function approach. 3rd ed. Philadelphia:Saunders; 2014.
24. Cole S, Gutnick D, Davis C, Cole M. Brief action planning (BAP): a self-management support tool. In: Bickley L. Bates’ guide to physical examination and history taking. 11th ed. Philadelphia: Lippincott Williams and Wilkins; 2013.
25. AMA Physician tip sheet for self-management support. Available at www.ama-assn.org/ama1/pub/upload/mm/433/phys_tip_sheet.pdf.
26. Taksler G, Keshner M, Fagerlin A. Personalized estimates of benefit from preventive care guidelines. Ann Intern Med 2013;159:161–9.
27. Centre for Comprehensive Motivational Interventions [website]. Available at www.centreecmi.com.
28. Del Canale S, Louis DZ, Maio V, et al. The relationship between physician empathy and disease complications: an empirical study of primary care physicians and their diabetic patients in Parma, Italy. Acad Med 2012;87:1243–9.
29. Moyers TB, Miller WR, Hendrickson SML. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. J Consult Clin Psychol 2005;73:590–8.
30. Hojat M, Louis DZ, Markham FW, et al. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64.
31. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
32. Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37:129–40.
33. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;85:191–215.
34. Kiesler, Charles A. The psychology of commitment: experiments linking behavior to belief. New York: Academic Press;1971.
35. Lorig K, Laurent DD, Plant K, et al. The components of action planning and their associations with behavior and health outcomes. Chronic Illn 2013.
36. MacGregor K, Handley M, Wong S, et al. Behavior-change action plans in primary care: a feasibility study of clinicians. J Am Board Fam Med 19:215–23.
37. Gollwitzer P. Implementation intentions. Am Psychol 1999;54:493–503.
38. Gollwitzer P, Sheeran P. Implementation intensions and goal achievement: A meta-analysis of effects and processes. Adv Exp Soc Psychology 2006;38:69–119.
39. Stretcher V, De Vellis B, Becker M, Rosenstock I. The role of self-efficacy in achieving behavior change. Health Educ Q 1986;13:73–92.
40. Ajzen I. Constructing a theory of planned behavior questionnaire. Available at people.umass.edu/aizen/pdf/tpb.measurement.pdf.
41. Rogers RW. Protection motivation theory of fear appeals and attitude-change. J Psychol 1975;91:93–114.
42. Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol An Int Rev 2008;57:1–29.
43. Cialdini R. Influence: science and practice. 5th ed. Boston:Allyn and Bacon; 2008.
44. Stott NC, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: diabetes care and negotiating skills. Fam Pract 1995;12:413–8.
45. Miller WR, Rollnick S, Butler C. Motivational interviewing in health care. New York: Guilford Press; 2008.
46. Bodenheimer T, Handley M. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns 2009;76:174–80.
47. Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. Am Psychol 2002;57:705–17.
48. Doran G. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev 1981;70:35–6.
49. Locke EA. Toward a theory of task motivation and incentives. Organ Behav Hum Perform 1968;3:157–89.
50. Locke EA, Latham GP, Erez M. The determinants of goal commitment. Acad Manag Rev 1988;13:23–39.
51. Lorig K, Homan H, Sobel D, et al. Living a healthy life with chronic conditions. 4th ed. Boulder: Bull Publishing; 2012.
52. Amrhein PC, Miller WR, Yahne CE, et al. Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003;71:862–78.
53. Ahaeonovich E, Amrhein PC, Bisaha A, et al. Cognition, commitment language and behavioral change among cocaine-dependent patients. Psychol Addict Behav 2008;22:557–62.
54. Gutnick D. Centre for Comprehensive Motivational Interventions community of practice webinar. Brief action planning and culture: developing culturally specific confidence rules. 2012. Available at www.centrecmi.ca.
55. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults. A scientific statement from the American Heart Association. Circulation 2010;122:406–41.
56. Handley M, MacGregor K, Schillinger D, et al. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med 2006;19:224–31.
57. Joint Commision. Primary care medical home option-additional requirements. Available at www.jointcommission.org/assets/1/18/PCMH_new_stds_by_5_characteristics.pdf.
58. Oregon Health Policy and Research. Standards for patient centered medical home recognition. Available at www.oregon.gov/oha/OHPR/pages/healthreform/pcpch/standards.aspx.
59. Nutting PA, Crabtree BF, Miller WL, et al. Journey to the patient-centered medical home: a qualitative analysis of the experiences of practices in the national demonstration project. Am Fam Med 2010;8(Suppl 1):S45–S56.
60. Stewart EE, Nutting PA, Crabtree BF, et al. Implementing the patient-centered medical home: observation and description of the National Demonstration Project. Am Fam Med 2010;8(Suppl 1):S21–S32.