User login
What Would I Tell My Intern-Year Self?
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
The training path to dermatology can seem interminable. From getting good grades in college to seeking out the “right” extracurricular activities and cramming for the MCAT, just getting into medical school was a huge challenge. In medical school, you may recognize the same chaos as you begin to prepare for US Medical Licensing Examination Step 1, try to volunteer, and publish original research. Dermatology is undeniably a competitive specialty. The 2018 data released by the National Resident Match Program (also called The Match) showed that only 83% of 412 US seniors who applied were matched to dermatology.1 The average Step 1 score for those who matched was 249 versus 241 for those who did not match. In addition, they had an average of 5.2 research experiences, 9.1 volunteer experiences, and 49.1 were members of Alpha Omega Alpha.1
After studying and working to meet these targets, it is not surprising that the transition to residency is a big change. As a dermatology preliminary intern, or“prelim,” our experience differs compared to other specialties, as other interns are jumping into their area of practice right away.
During my intern year, I had a tremendous amount of anxiety about 2 things: (1) being a subpar medical intern and (2) being unprepared for the beginning of my dermatology residency. This anxiety drove me to read a tremendous amount of medical and dermatological literature in an effort to do everything. Although hindsight is always 20/20, I will share some thoughts of my own as well as some from friends and colleagues.
First, enjoy intern year. I know that may sound ridiculous, but there were many aspects of intern year that I loved! When your pager beeps, it’s for YOU! You are no longer a subintern, running every decision past your intern or explaining your student status to the patients! Proudly introduce yourself as Dr. So-and-So. You earned it! I loved the camaraderie of working with my co-interns and senior residents. Going through the challenges of intern year together is a deep bonding experience, and I absolutely made lifelong friendships. It also does not hurt that I met my boyfriend (now husband), which has changed my life in a big way.
When it comes to learning internal medicine, pediatrics, or surgery (depending on your intern year), prepare for rounds, read about your patients, and pay attention in Grand Rounds. You can even consider taking the dermatologic cases that may be on your team, just for fun. I am always grateful for my internal medicine knowledge when managing complex medical dermatology patients and rounding on our consultation service on the wards. However, do not burden yourself with excessive studying. Enjoy your time off: spend it with family and friends or rediscover a hobby that has been neglected while you have been working toward your achievements.
When it comes to learning dermatology, do not rush it! You have 3 years and a ton of studying ahead of you! You will learn all of it. When July 1 of your first year of dermatology finally starts, immerse yourself in this new world:
- Attend conferences. Even if they are on topics you might not be interested in—from cosmetics to psoriasis—they provide a real-world perspective and often have great lecturers sharing their knowledge.
- Get involved. There are many dermatologic societies to take part in, and dues are waived or reduced when you sign up as a resident. Many of them provide great resources from study materials to journals, and they are always a great way to network when there are events.
- Volunteer. Many of the dermatologic societies sponsor volunteer events such as skin cancer screenings. It can be a fun way to network while also giving back to the community.
- Spend time figuring out what you really enjoy. This step may seem self-evident, but after many years of fulfilling the necessary criteria to get into medical school and residency, it can be habitual to start fulfilling the same criteria all over again. Explore all aspects of dermatology and see what truly interests you. Consider how you expect your life after residency to be and think what learning opportunities might be helpful down the road. Reach out to attendings you would like to work with, both in dermatology and in other specialties. I personally enjoyed working in wound and oncology clinics, learning how other specialties approach clinical dilemmas that we see in dermatology.
As I embark on my final year of dermatology residency, I am truly grateful for the wisdom that has been shared with me on this journey. Many people have provided key pieces of information that have helped shape my training and my plans for the future, and I hope that sharing it will help others!
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
- National Resident Matching Program, Charting Outcomes in the Match: U.S. Allopathic Seniors, 2018. Washington, DC: National Resident Matching Program; 2018. http://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed September 20, 2018.
Bedside Microscopy for the Beginner
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
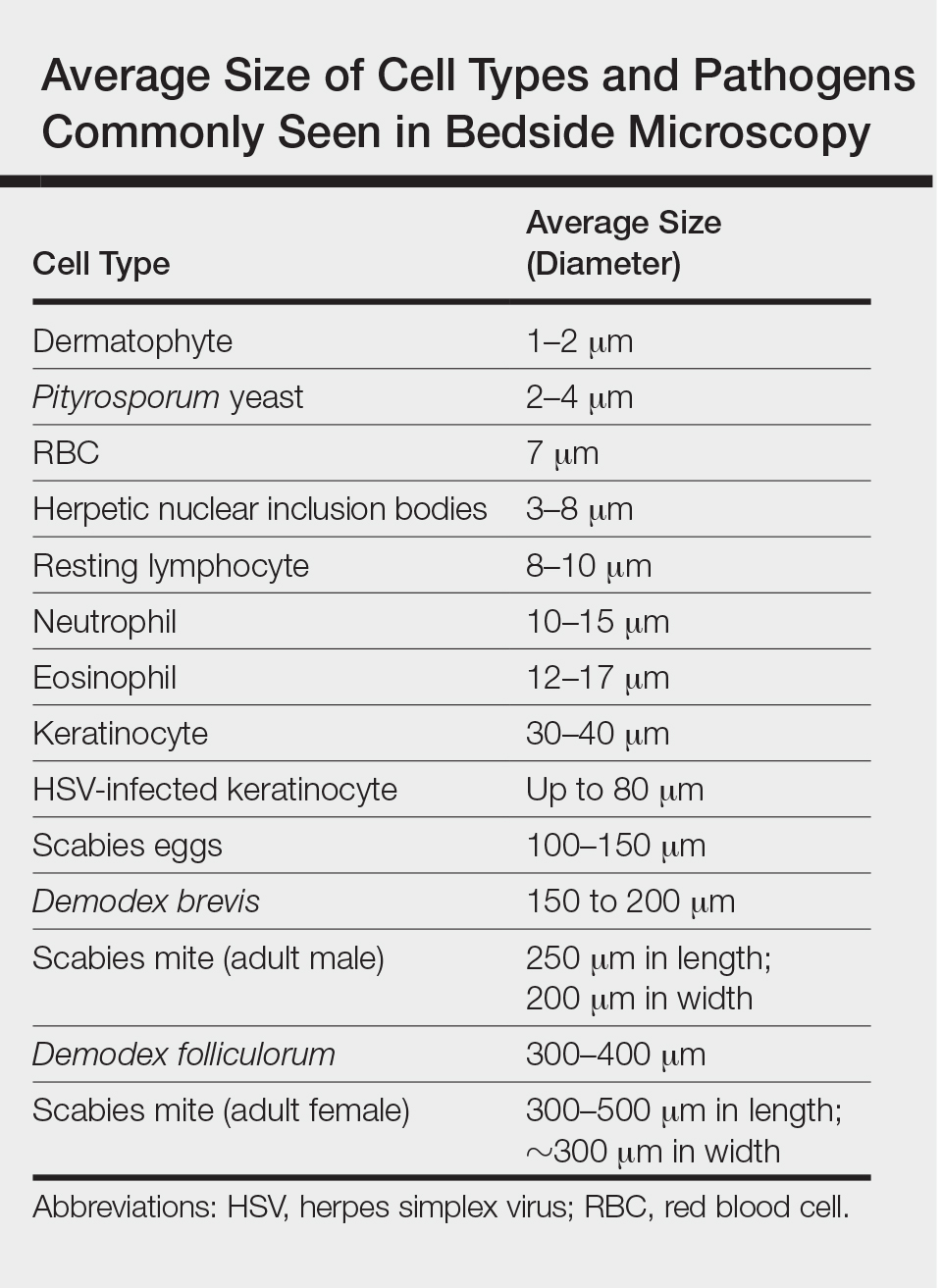
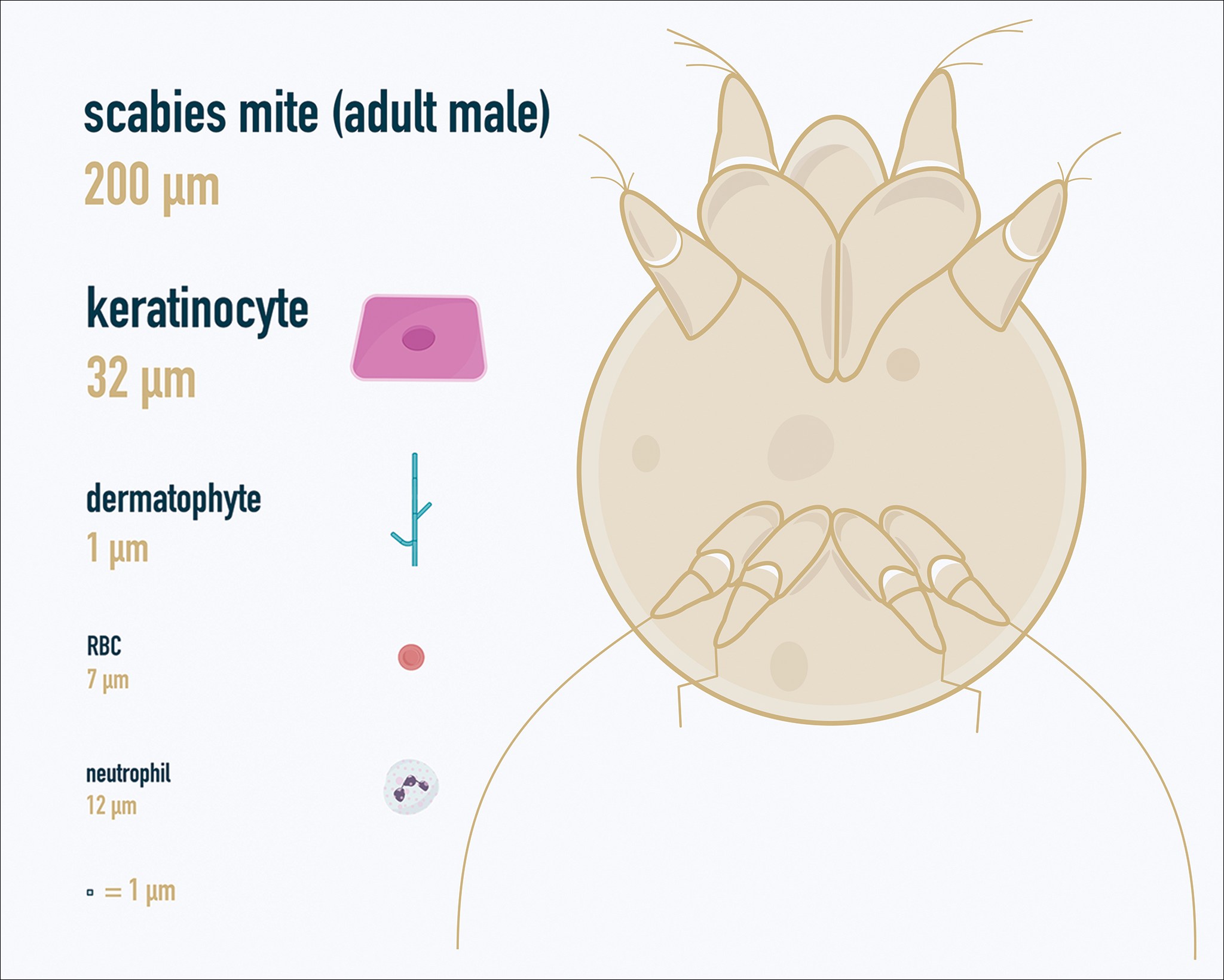
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.
Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
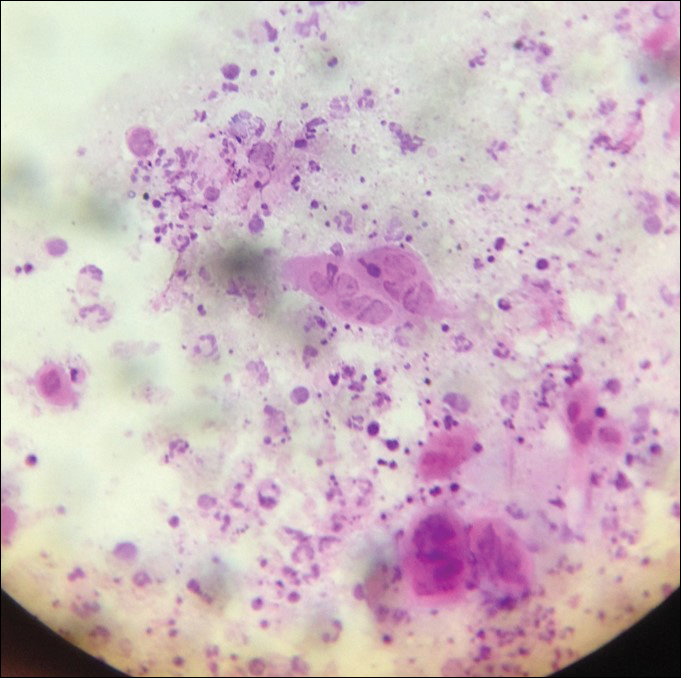
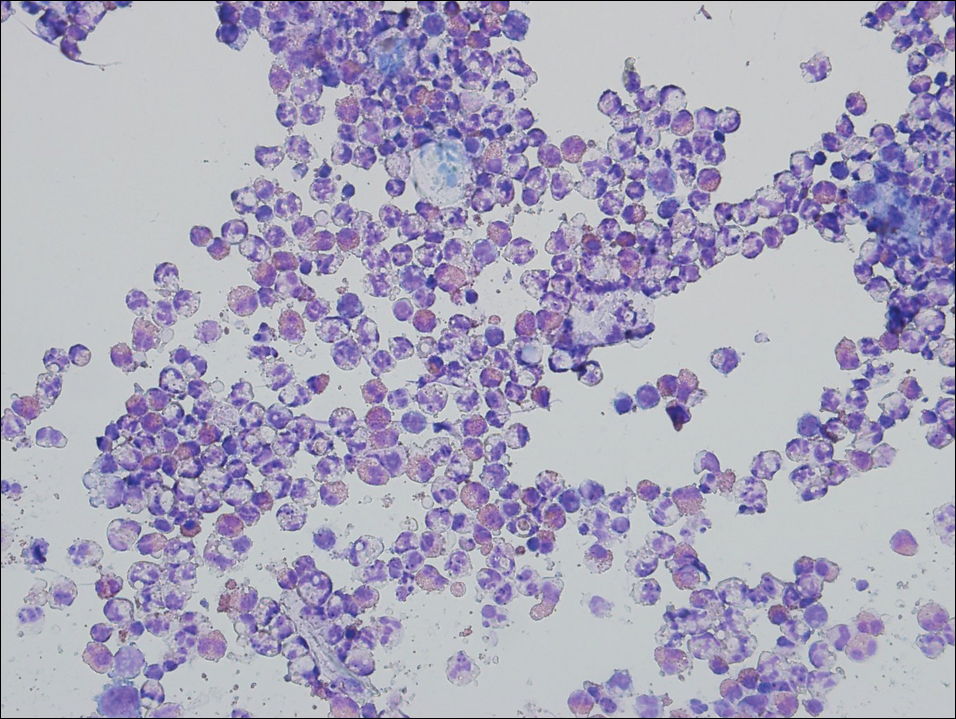
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2
Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12
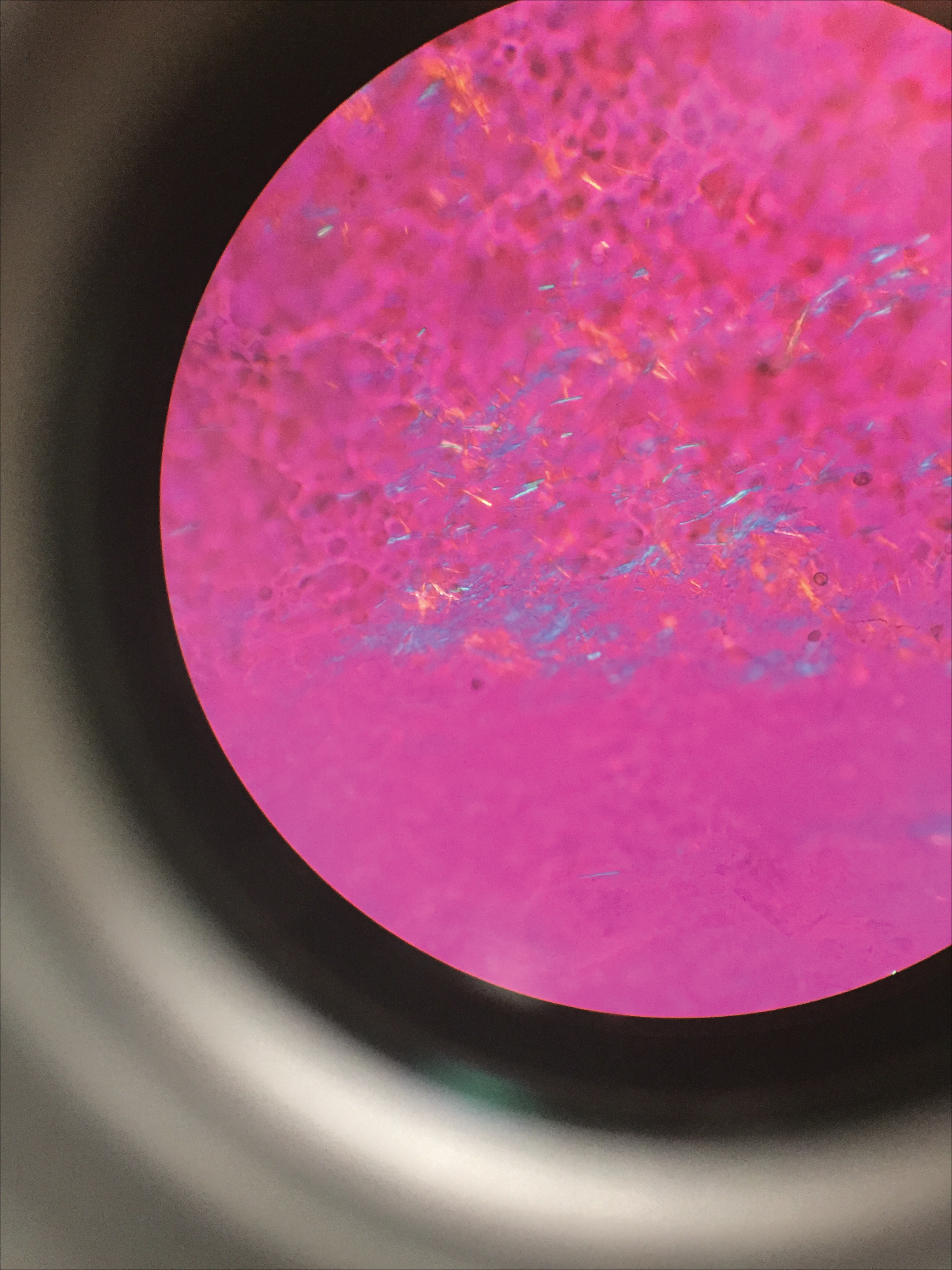
Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.
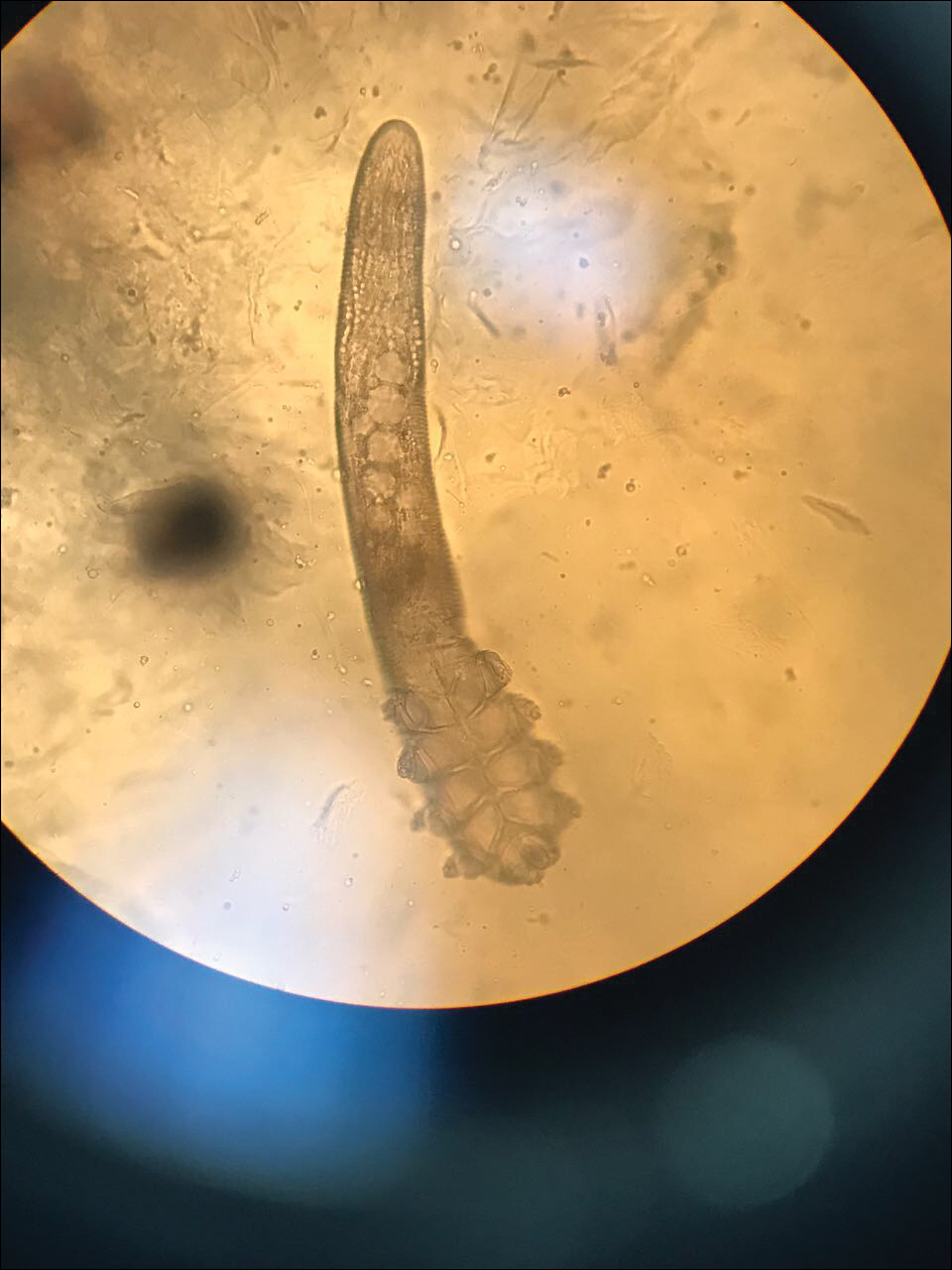
Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.

Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.

Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Physician Burnout in Dermatology
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
Tattoos: From Ancient Practice to Modern Treatment Dilemma
As dermatologists, we possess a vast knowledge of the epidermis. Some patients may choose to use the epidermis as a canvas for their art in the form of tattoos; however, tattoos can complicate dermatology visits in a myriad of ways. From patients seeking tattoo removal (a complicated task even with the most advanced laser treatments) to those whose native skin is obscured by a tattoo during melanoma screening, it is no wonder that many dermatologists become frustrated at the very mention of the word tattoo.
Tattoos have a long and complicated history entrenched in class divisions, gender identity, and culture. Although its origins are not well documented, many researchers believe that tattooing began in Egypt as early as 4000 BCE.1 From there, the practice spread east into South Asia and west to the British Isles and Scotland. The Iberians in the British Isles, the Picts in Scotland, the Gauls in Western Europe, and the Teutons in Germany all practiced tattooing, and the Romans were known to use tattooing to mark convicts and slaves.1 By 787 AD, tattooing was prevalent enough to warrant an official ban by Pope Hadrian I at the Second Ecumenical Council of Nicaea.2 The growing power of Christianity most likely contributed to the elimination of tattooing in the West, although many soldiers who fought in the Crusades received tattoos during their travels.3
Despite the long history of tattoos in both the East and West, Captain James Cook often is credited with discovering tattooing in the eighteenth century during his explorations in the Pacific.4 In Tahiti in 1769 and Hawaii in 1778, Cook encountered heavily tattooed populations who deposited dye into the skin by tapping sharpened instruments.3 These Polynesian tattoos, which were associated with healing and protective powers, often depicted genealogies and were composed of images of lines, stars, geometric designs, animals, and humans. Explorers in Polynesia who came after Cook noted that tattoo designs began to include rifles, cannons, and dates of chief’s deaths—an indication of the cultural exchange that occurred between Cook’s crew and the natives.3 The first tattooed peoples were displayed in the United States at the Centennial Exhibition in Philadelphia, Pennsylvania, in 1876.2 Later, at the 1901 World’s Fair in Buffalo, New York, the first full “freak show” emerged, and tattooed “natives” were displayed.5 Since they were introduced in the West, tattoos have been associated with an element of the exotic in the United States.
Acknowledged by many to be the first professional tattooist in the United States, Martin Hildebrandt opened his shop in New York City, New York, in 1846.2 Initially, only sailors and soldiers were tattooed, which contributed to the concept of the so-called “tattooed serviceman.”5 However, after the Spanish-American War, tattoos became a fad among the high society in Europe. Tattooing at this time was still performed through the ancient Polynesian tapping method, making it both time-consuming and expensive. Tattoos generally were always placed in a private location, leading to popular speculation at the time about whom in the aristocracy possessed a tattoo, with some even speculating that Queen Victoria may have had a tattoo.1 However, this brief trend among the aristocracy came to an end when Samuel O’Reilly, an American tattoo artist, patented the first electric tattooing machine in 1891.6 His invention made tattooing faster, cheaper, and less painful, thereby making tattooing available to a much wider audience. In the United States, men in the military often were tattooed, especially during World Wars I and II, when patriotic themes and tattoos of important women in their lives (eg, the word Mom, the name of a sweetheart) became popular.
It is a popular belief that a tattoo renaissance occurred in the United States in the 1970s, sparked by an influx of Indonesian and Asian artistic styles. Today, tattoos are ubiquitous. A 2012 poll showed that 21% of adults in the United States have a tattoo.7 There are now 4 main types of tattoos: cosmetic (eg, permanent makeup), traumatic (eg, injury on asphalt), medical (eg, to mark radiation sites), and decorative—either amateur (often done by hand) or professional (done in tattoo parlors with electric tattooing needles).8
Laser Tattoo Removal
Today tattoos are easy and relatively cheap to get, and for most people they are not regarded as an important cultural milestone like they were in early Polynesian culture. As a result, dermatologists often may encounter patients seeking to have these permanent designs removed from their skin. Previously, tattoo removal was attempted using destructive processes such as scarification and cryotherapy and generally resulted in poor cosmetics outcomes. Today, lasers are at the forefront of tattoo removal. Traditional lasers use pulse durations in the nanosecond range, with newer generation lasers in the picosecond range delivering much shorter pulse durations, effectively delivering the same level of energy over less time. It is important to select the correct laser for optimal destruction of various tattoo ink colors (Table).8,9
Controversy persists as to whether tattoo pigment destruction by lasers is caused by thermal or acoustic damage.10 It may be a combination of both, with rapid heating of the particles leading to a local shockwave as the energy collapses.11 The goal of tattoo removal is to create smaller granules of pigment that can be taken up by the patient’s lymphatic system. The largest granule that can be taken up by the lymphatic system is 0.4 μm.10
In laser treatment of any skin condition, the laser energy is delivered in a pulse duration that should be less than the thermal relaxation time of the chromophores (water, melanin, hemoglobin, or tattoo pigment are the main targets within the skin).12 Most tattoo chromophores are 30 nm to 300 nm, with a thermal relaxation time of less than 10 nanoseconds.10,12 As the number of treatments progresses, laser settings should be adjusted for smaller ink particles. Patients should be warned about pain, side effects, and the need for multiple treatments. Common side effects of laser tattoo removal include purpura, pinpoint bleeding, erythema, edema, crusting, and blistering.8
After laser treatment, cytoplasmic water in the cell is converted into steam leading to cavitation of the lysosome, which presents as whitening of the skin. The whitening causes optical scatter, thereby preventing immediate retreatment of the area.11 The R20 laser tattoo removal method discussed by Kossida et al,13 advises practitioners to wait 20 minutes between treatments to allow the air bubbles from the conversion of water to steam to disappear. Kossida et al13 demonstrated more effective removal in tattoos that were treated with this method compared to standard treatment. The recognition that trapped air bubbles delay multiple treatment cycles has led to the experimental use of perfluorodecalin, a fluorocarbon liquid capable of dissolving the air bubbles, for immediate retreatment.14 By dissolving the trapped air and eliminating the white color, multiple treatments can be completed during 1 session.
Risks of Laser Tattoo Removal
It is important to emphasize that there are potential risks associated with laser treatment for tattoo removal, many of which we are only just beginning to understand. Common side effects of laser treatment for tattoo removal include blisters, pain, bleeding, hyperpigmentation, or hypopigmentation; however, there also are rare potential risks. Tattoo ink can paradoxically darken when it contains metals such as titanium or zinc, as often is found in tan or white inks.15 The laser energy causes a shift of the metal from an oxidized to a reduced state, leading to a darker rather than lighter tattoo upon application of the laser. There also have been documented cases of intraprocedural anaphylaxis, delayed urticaria, as well as generalized eczematous reactions.16-18 In these cases, the patients had never experienced any allergic symptoms prior to the laser tattoo removal procedure.
Additionally, patients with active allergy to the pigments used in tattoo ink provide a therapeutic dilemma, as laser treatment may potentially systematize the tattoo ink, leading to a more widespread allergic reaction. A case of a generalized eczematous reaction after carbon dioxide laser therapy in a patient with documented tattoo allergy has been reported.19 More research is needed to fully understand the nature of immediate as well as delayed hypersensitivity reactions associated with laser tattoo removal.
Final Thoughts
With thousands of years of established traditions, it is unlikely that tattooing will go away anytime soon. Fortunately, lasers are providing us with an effective and safe method of removal.
- Caplan J, ed. Written on the Body: The Tattoo in European and American History. Princeton, NJ: Princeton University Press; 2000.
- DeMello M. Bodies of Inscription: Cultural History of the Modern Tattoo Community. Durham, NC: Duke University Press; 2000.
- DeMello M. “Not just for bikers anymore”: popular representations of american tattooing. J Popular Culture. 1995;29:37-52.
- Anastasia DJM. Living marked: tattooed women and perceptions of beauty and femininity. In: Segal MT, ed. Interactions and Intersections of Gendered Bodies at Work, at Home, and at Play. Bingly, UK: Emerald; 2010.
- Mifflin M. Bodies of Subversion: A Secret History of Women and Tattoo. New York: June Books; 1997.
- Atkinson M. Pretty in ink: conformity, resistance, and negotiation in women’s tattooing. Sex Roles. 2002;47:219-235.
- Braverman S. One in five US adults now has a tattoo. Harris Poll website. https://theharrispoll.com/new-york-n-y-february-23-2012-there-is-a-lot-of-culture-and-lore-associated-with-tattoos-from-ancient-art-to-modern-expressionism-and-there-are-many-reasons-people-choose-to-get-or-not-get-p/. Published February 23, 2012. Accessed May 25, 2018.
- Ho SG, Goh CL. Laser tattoo removal: a clinical update. J Cutan Aesthet Surg. 2015;8:9-15.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24.
- Shah SD, Aurangabadkar SJ. Newer trends in laser tattoo removal. J Cutan Aesthet Surg. 2015;8:25-29.
- Hsu VM, Aldahan AS, Mlacker S, et al. The picosecond laser for tattoo removal. Lasers Med Sci. 2016;31:1733-1737.
- Kossida T, Rigopoulos D, Katsambas A, et al. Optimal tattoo removal in a single laser session based on the method of repeated exposures.J Am Acad Dermatol. 2012;66:271-277.
- Biesman BS, O’Neil MP, Costner C. Rapid, high-fluence multipass Q-switched laser treatment of tattoos with a transparent perfluorodecalin-infused patch: a pilot study. Lasers Surg Med. 2015;47:613-618.
- Bernstein EF. Laser tattoo removal. Semin Plast Surg. 2007;21:175-192.
- Wilken R, Ho D, Petukhova T, et al. Intraoperative localized urticarial reaction during Q-switched Nd:YAG laser tattoo removal. J Drugs Dermatol. 2015;14:303-306.
- Hibler BP, Rossi AM. A case of delayed anaphylaxis after laser tattoo removal. JAAD Case Rep. 2015;1:80-81.
- Bernstein EF. A widespread allergic reaction to black tattoo ink caused by laser treatment. Lasers Surg Med. 2015;47:180-182.
- Meesters AA, De Rie MA, Wolkerstorfer A. Generalized eczematous reaction after fractional carbon dioxide laser therapy for tattoo allergy. J Cosmet Laser Ther. 2016;18:456-458.
As dermatologists, we possess a vast knowledge of the epidermis. Some patients may choose to use the epidermis as a canvas for their art in the form of tattoos; however, tattoos can complicate dermatology visits in a myriad of ways. From patients seeking tattoo removal (a complicated task even with the most advanced laser treatments) to those whose native skin is obscured by a tattoo during melanoma screening, it is no wonder that many dermatologists become frustrated at the very mention of the word tattoo.
Tattoos have a long and complicated history entrenched in class divisions, gender identity, and culture. Although its origins are not well documented, many researchers believe that tattooing began in Egypt as early as 4000 BCE.1 From there, the practice spread east into South Asia and west to the British Isles and Scotland. The Iberians in the British Isles, the Picts in Scotland, the Gauls in Western Europe, and the Teutons in Germany all practiced tattooing, and the Romans were known to use tattooing to mark convicts and slaves.1 By 787 AD, tattooing was prevalent enough to warrant an official ban by Pope Hadrian I at the Second Ecumenical Council of Nicaea.2 The growing power of Christianity most likely contributed to the elimination of tattooing in the West, although many soldiers who fought in the Crusades received tattoos during their travels.3
Despite the long history of tattoos in both the East and West, Captain James Cook often is credited with discovering tattooing in the eighteenth century during his explorations in the Pacific.4 In Tahiti in 1769 and Hawaii in 1778, Cook encountered heavily tattooed populations who deposited dye into the skin by tapping sharpened instruments.3 These Polynesian tattoos, which were associated with healing and protective powers, often depicted genealogies and were composed of images of lines, stars, geometric designs, animals, and humans. Explorers in Polynesia who came after Cook noted that tattoo designs began to include rifles, cannons, and dates of chief’s deaths—an indication of the cultural exchange that occurred between Cook’s crew and the natives.3 The first tattooed peoples were displayed in the United States at the Centennial Exhibition in Philadelphia, Pennsylvania, in 1876.2 Later, at the 1901 World’s Fair in Buffalo, New York, the first full “freak show” emerged, and tattooed “natives” were displayed.5 Since they were introduced in the West, tattoos have been associated with an element of the exotic in the United States.
Acknowledged by many to be the first professional tattooist in the United States, Martin Hildebrandt opened his shop in New York City, New York, in 1846.2 Initially, only sailors and soldiers were tattooed, which contributed to the concept of the so-called “tattooed serviceman.”5 However, after the Spanish-American War, tattoos became a fad among the high society in Europe. Tattooing at this time was still performed through the ancient Polynesian tapping method, making it both time-consuming and expensive. Tattoos generally were always placed in a private location, leading to popular speculation at the time about whom in the aristocracy possessed a tattoo, with some even speculating that Queen Victoria may have had a tattoo.1 However, this brief trend among the aristocracy came to an end when Samuel O’Reilly, an American tattoo artist, patented the first electric tattooing machine in 1891.6 His invention made tattooing faster, cheaper, and less painful, thereby making tattooing available to a much wider audience. In the United States, men in the military often were tattooed, especially during World Wars I and II, when patriotic themes and tattoos of important women in their lives (eg, the word Mom, the name of a sweetheart) became popular.
It is a popular belief that a tattoo renaissance occurred in the United States in the 1970s, sparked by an influx of Indonesian and Asian artistic styles. Today, tattoos are ubiquitous. A 2012 poll showed that 21% of adults in the United States have a tattoo.7 There are now 4 main types of tattoos: cosmetic (eg, permanent makeup), traumatic (eg, injury on asphalt), medical (eg, to mark radiation sites), and decorative—either amateur (often done by hand) or professional (done in tattoo parlors with electric tattooing needles).8
Laser Tattoo Removal
Today tattoos are easy and relatively cheap to get, and for most people they are not regarded as an important cultural milestone like they were in early Polynesian culture. As a result, dermatologists often may encounter patients seeking to have these permanent designs removed from their skin. Previously, tattoo removal was attempted using destructive processes such as scarification and cryotherapy and generally resulted in poor cosmetics outcomes. Today, lasers are at the forefront of tattoo removal. Traditional lasers use pulse durations in the nanosecond range, with newer generation lasers in the picosecond range delivering much shorter pulse durations, effectively delivering the same level of energy over less time. It is important to select the correct laser for optimal destruction of various tattoo ink colors (Table).8,9
Controversy persists as to whether tattoo pigment destruction by lasers is caused by thermal or acoustic damage.10 It may be a combination of both, with rapid heating of the particles leading to a local shockwave as the energy collapses.11 The goal of tattoo removal is to create smaller granules of pigment that can be taken up by the patient’s lymphatic system. The largest granule that can be taken up by the lymphatic system is 0.4 μm.10
In laser treatment of any skin condition, the laser energy is delivered in a pulse duration that should be less than the thermal relaxation time of the chromophores (water, melanin, hemoglobin, or tattoo pigment are the main targets within the skin).12 Most tattoo chromophores are 30 nm to 300 nm, with a thermal relaxation time of less than 10 nanoseconds.10,12 As the number of treatments progresses, laser settings should be adjusted for smaller ink particles. Patients should be warned about pain, side effects, and the need for multiple treatments. Common side effects of laser tattoo removal include purpura, pinpoint bleeding, erythema, edema, crusting, and blistering.8
After laser treatment, cytoplasmic water in the cell is converted into steam leading to cavitation of the lysosome, which presents as whitening of the skin. The whitening causes optical scatter, thereby preventing immediate retreatment of the area.11 The R20 laser tattoo removal method discussed by Kossida et al,13 advises practitioners to wait 20 minutes between treatments to allow the air bubbles from the conversion of water to steam to disappear. Kossida et al13 demonstrated more effective removal in tattoos that were treated with this method compared to standard treatment. The recognition that trapped air bubbles delay multiple treatment cycles has led to the experimental use of perfluorodecalin, a fluorocarbon liquid capable of dissolving the air bubbles, for immediate retreatment.14 By dissolving the trapped air and eliminating the white color, multiple treatments can be completed during 1 session.
Risks of Laser Tattoo Removal
It is important to emphasize that there are potential risks associated with laser treatment for tattoo removal, many of which we are only just beginning to understand. Common side effects of laser treatment for tattoo removal include blisters, pain, bleeding, hyperpigmentation, or hypopigmentation; however, there also are rare potential risks. Tattoo ink can paradoxically darken when it contains metals such as titanium or zinc, as often is found in tan or white inks.15 The laser energy causes a shift of the metal from an oxidized to a reduced state, leading to a darker rather than lighter tattoo upon application of the laser. There also have been documented cases of intraprocedural anaphylaxis, delayed urticaria, as well as generalized eczematous reactions.16-18 In these cases, the patients had never experienced any allergic symptoms prior to the laser tattoo removal procedure.
Additionally, patients with active allergy to the pigments used in tattoo ink provide a therapeutic dilemma, as laser treatment may potentially systematize the tattoo ink, leading to a more widespread allergic reaction. A case of a generalized eczematous reaction after carbon dioxide laser therapy in a patient with documented tattoo allergy has been reported.19 More research is needed to fully understand the nature of immediate as well as delayed hypersensitivity reactions associated with laser tattoo removal.
Final Thoughts
With thousands of years of established traditions, it is unlikely that tattooing will go away anytime soon. Fortunately, lasers are providing us with an effective and safe method of removal.
As dermatologists, we possess a vast knowledge of the epidermis. Some patients may choose to use the epidermis as a canvas for their art in the form of tattoos; however, tattoos can complicate dermatology visits in a myriad of ways. From patients seeking tattoo removal (a complicated task even with the most advanced laser treatments) to those whose native skin is obscured by a tattoo during melanoma screening, it is no wonder that many dermatologists become frustrated at the very mention of the word tattoo.
Tattoos have a long and complicated history entrenched in class divisions, gender identity, and culture. Although its origins are not well documented, many researchers believe that tattooing began in Egypt as early as 4000 BCE.1 From there, the practice spread east into South Asia and west to the British Isles and Scotland. The Iberians in the British Isles, the Picts in Scotland, the Gauls in Western Europe, and the Teutons in Germany all practiced tattooing, and the Romans were known to use tattooing to mark convicts and slaves.1 By 787 AD, tattooing was prevalent enough to warrant an official ban by Pope Hadrian I at the Second Ecumenical Council of Nicaea.2 The growing power of Christianity most likely contributed to the elimination of tattooing in the West, although many soldiers who fought in the Crusades received tattoos during their travels.3
Despite the long history of tattoos in both the East and West, Captain James Cook often is credited with discovering tattooing in the eighteenth century during his explorations in the Pacific.4 In Tahiti in 1769 and Hawaii in 1778, Cook encountered heavily tattooed populations who deposited dye into the skin by tapping sharpened instruments.3 These Polynesian tattoos, which were associated with healing and protective powers, often depicted genealogies and were composed of images of lines, stars, geometric designs, animals, and humans. Explorers in Polynesia who came after Cook noted that tattoo designs began to include rifles, cannons, and dates of chief’s deaths—an indication of the cultural exchange that occurred between Cook’s crew and the natives.3 The first tattooed peoples were displayed in the United States at the Centennial Exhibition in Philadelphia, Pennsylvania, in 1876.2 Later, at the 1901 World’s Fair in Buffalo, New York, the first full “freak show” emerged, and tattooed “natives” were displayed.5 Since they were introduced in the West, tattoos have been associated with an element of the exotic in the United States.
Acknowledged by many to be the first professional tattooist in the United States, Martin Hildebrandt opened his shop in New York City, New York, in 1846.2 Initially, only sailors and soldiers were tattooed, which contributed to the concept of the so-called “tattooed serviceman.”5 However, after the Spanish-American War, tattoos became a fad among the high society in Europe. Tattooing at this time was still performed through the ancient Polynesian tapping method, making it both time-consuming and expensive. Tattoos generally were always placed in a private location, leading to popular speculation at the time about whom in the aristocracy possessed a tattoo, with some even speculating that Queen Victoria may have had a tattoo.1 However, this brief trend among the aristocracy came to an end when Samuel O’Reilly, an American tattoo artist, patented the first electric tattooing machine in 1891.6 His invention made tattooing faster, cheaper, and less painful, thereby making tattooing available to a much wider audience. In the United States, men in the military often were tattooed, especially during World Wars I and II, when patriotic themes and tattoos of important women in their lives (eg, the word Mom, the name of a sweetheart) became popular.
It is a popular belief that a tattoo renaissance occurred in the United States in the 1970s, sparked by an influx of Indonesian and Asian artistic styles. Today, tattoos are ubiquitous. A 2012 poll showed that 21% of adults in the United States have a tattoo.7 There are now 4 main types of tattoos: cosmetic (eg, permanent makeup), traumatic (eg, injury on asphalt), medical (eg, to mark radiation sites), and decorative—either amateur (often done by hand) or professional (done in tattoo parlors with electric tattooing needles).8
Laser Tattoo Removal
Today tattoos are easy and relatively cheap to get, and for most people they are not regarded as an important cultural milestone like they were in early Polynesian culture. As a result, dermatologists often may encounter patients seeking to have these permanent designs removed from their skin. Previously, tattoo removal was attempted using destructive processes such as scarification and cryotherapy and generally resulted in poor cosmetics outcomes. Today, lasers are at the forefront of tattoo removal. Traditional lasers use pulse durations in the nanosecond range, with newer generation lasers in the picosecond range delivering much shorter pulse durations, effectively delivering the same level of energy over less time. It is important to select the correct laser for optimal destruction of various tattoo ink colors (Table).8,9
Controversy persists as to whether tattoo pigment destruction by lasers is caused by thermal or acoustic damage.10 It may be a combination of both, with rapid heating of the particles leading to a local shockwave as the energy collapses.11 The goal of tattoo removal is to create smaller granules of pigment that can be taken up by the patient’s lymphatic system. The largest granule that can be taken up by the lymphatic system is 0.4 μm.10
In laser treatment of any skin condition, the laser energy is delivered in a pulse duration that should be less than the thermal relaxation time of the chromophores (water, melanin, hemoglobin, or tattoo pigment are the main targets within the skin).12 Most tattoo chromophores are 30 nm to 300 nm, with a thermal relaxation time of less than 10 nanoseconds.10,12 As the number of treatments progresses, laser settings should be adjusted for smaller ink particles. Patients should be warned about pain, side effects, and the need for multiple treatments. Common side effects of laser tattoo removal include purpura, pinpoint bleeding, erythema, edema, crusting, and blistering.8
After laser treatment, cytoplasmic water in the cell is converted into steam leading to cavitation of the lysosome, which presents as whitening of the skin. The whitening causes optical scatter, thereby preventing immediate retreatment of the area.11 The R20 laser tattoo removal method discussed by Kossida et al,13 advises practitioners to wait 20 minutes between treatments to allow the air bubbles from the conversion of water to steam to disappear. Kossida et al13 demonstrated more effective removal in tattoos that were treated with this method compared to standard treatment. The recognition that trapped air bubbles delay multiple treatment cycles has led to the experimental use of perfluorodecalin, a fluorocarbon liquid capable of dissolving the air bubbles, for immediate retreatment.14 By dissolving the trapped air and eliminating the white color, multiple treatments can be completed during 1 session.
Risks of Laser Tattoo Removal
It is important to emphasize that there are potential risks associated with laser treatment for tattoo removal, many of which we are only just beginning to understand. Common side effects of laser treatment for tattoo removal include blisters, pain, bleeding, hyperpigmentation, or hypopigmentation; however, there also are rare potential risks. Tattoo ink can paradoxically darken when it contains metals such as titanium or zinc, as often is found in tan or white inks.15 The laser energy causes a shift of the metal from an oxidized to a reduced state, leading to a darker rather than lighter tattoo upon application of the laser. There also have been documented cases of intraprocedural anaphylaxis, delayed urticaria, as well as generalized eczematous reactions.16-18 In these cases, the patients had never experienced any allergic symptoms prior to the laser tattoo removal procedure.
Additionally, patients with active allergy to the pigments used in tattoo ink provide a therapeutic dilemma, as laser treatment may potentially systematize the tattoo ink, leading to a more widespread allergic reaction. A case of a generalized eczematous reaction after carbon dioxide laser therapy in a patient with documented tattoo allergy has been reported.19 More research is needed to fully understand the nature of immediate as well as delayed hypersensitivity reactions associated with laser tattoo removal.
Final Thoughts
With thousands of years of established traditions, it is unlikely that tattooing will go away anytime soon. Fortunately, lasers are providing us with an effective and safe method of removal.
- Caplan J, ed. Written on the Body: The Tattoo in European and American History. Princeton, NJ: Princeton University Press; 2000.
- DeMello M. Bodies of Inscription: Cultural History of the Modern Tattoo Community. Durham, NC: Duke University Press; 2000.
- DeMello M. “Not just for bikers anymore”: popular representations of american tattooing. J Popular Culture. 1995;29:37-52.
- Anastasia DJM. Living marked: tattooed women and perceptions of beauty and femininity. In: Segal MT, ed. Interactions and Intersections of Gendered Bodies at Work, at Home, and at Play. Bingly, UK: Emerald; 2010.
- Mifflin M. Bodies of Subversion: A Secret History of Women and Tattoo. New York: June Books; 1997.
- Atkinson M. Pretty in ink: conformity, resistance, and negotiation in women’s tattooing. Sex Roles. 2002;47:219-235.
- Braverman S. One in five US adults now has a tattoo. Harris Poll website. https://theharrispoll.com/new-york-n-y-february-23-2012-there-is-a-lot-of-culture-and-lore-associated-with-tattoos-from-ancient-art-to-modern-expressionism-and-there-are-many-reasons-people-choose-to-get-or-not-get-p/. Published February 23, 2012. Accessed May 25, 2018.
- Ho SG, Goh CL. Laser tattoo removal: a clinical update. J Cutan Aesthet Surg. 2015;8:9-15.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24.
- Shah SD, Aurangabadkar SJ. Newer trends in laser tattoo removal. J Cutan Aesthet Surg. 2015;8:25-29.
- Hsu VM, Aldahan AS, Mlacker S, et al. The picosecond laser for tattoo removal. Lasers Med Sci. 2016;31:1733-1737.
- Kossida T, Rigopoulos D, Katsambas A, et al. Optimal tattoo removal in a single laser session based on the method of repeated exposures.J Am Acad Dermatol. 2012;66:271-277.
- Biesman BS, O’Neil MP, Costner C. Rapid, high-fluence multipass Q-switched laser treatment of tattoos with a transparent perfluorodecalin-infused patch: a pilot study. Lasers Surg Med. 2015;47:613-618.
- Bernstein EF. Laser tattoo removal. Semin Plast Surg. 2007;21:175-192.
- Wilken R, Ho D, Petukhova T, et al. Intraoperative localized urticarial reaction during Q-switched Nd:YAG laser tattoo removal. J Drugs Dermatol. 2015;14:303-306.
- Hibler BP, Rossi AM. A case of delayed anaphylaxis after laser tattoo removal. JAAD Case Rep. 2015;1:80-81.
- Bernstein EF. A widespread allergic reaction to black tattoo ink caused by laser treatment. Lasers Surg Med. 2015;47:180-182.
- Meesters AA, De Rie MA, Wolkerstorfer A. Generalized eczematous reaction after fractional carbon dioxide laser therapy for tattoo allergy. J Cosmet Laser Ther. 2016;18:456-458.
- Caplan J, ed. Written on the Body: The Tattoo in European and American History. Princeton, NJ: Princeton University Press; 2000.
- DeMello M. Bodies of Inscription: Cultural History of the Modern Tattoo Community. Durham, NC: Duke University Press; 2000.
- DeMello M. “Not just for bikers anymore”: popular representations of american tattooing. J Popular Culture. 1995;29:37-52.
- Anastasia DJM. Living marked: tattooed women and perceptions of beauty and femininity. In: Segal MT, ed. Interactions and Intersections of Gendered Bodies at Work, at Home, and at Play. Bingly, UK: Emerald; 2010.
- Mifflin M. Bodies of Subversion: A Secret History of Women and Tattoo. New York: June Books; 1997.
- Atkinson M. Pretty in ink: conformity, resistance, and negotiation in women’s tattooing. Sex Roles. 2002;47:219-235.
- Braverman S. One in five US adults now has a tattoo. Harris Poll website. https://theharrispoll.com/new-york-n-y-february-23-2012-there-is-a-lot-of-culture-and-lore-associated-with-tattoos-from-ancient-art-to-modern-expressionism-and-there-are-many-reasons-people-choose-to-get-or-not-get-p/. Published February 23, 2012. Accessed May 25, 2018.
- Ho SG, Goh CL. Laser tattoo removal: a clinical update. J Cutan Aesthet Surg. 2015;8:9-15.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. China: Elsevier Saunders; 2012.
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24.
- Shah SD, Aurangabadkar SJ. Newer trends in laser tattoo removal. J Cutan Aesthet Surg. 2015;8:25-29.
- Hsu VM, Aldahan AS, Mlacker S, et al. The picosecond laser for tattoo removal. Lasers Med Sci. 2016;31:1733-1737.
- Kossida T, Rigopoulos D, Katsambas A, et al. Optimal tattoo removal in a single laser session based on the method of repeated exposures.J Am Acad Dermatol. 2012;66:271-277.
- Biesman BS, O’Neil MP, Costner C. Rapid, high-fluence multipass Q-switched laser treatment of tattoos with a transparent perfluorodecalin-infused patch: a pilot study. Lasers Surg Med. 2015;47:613-618.
- Bernstein EF. Laser tattoo removal. Semin Plast Surg. 2007;21:175-192.
- Wilken R, Ho D, Petukhova T, et al. Intraoperative localized urticarial reaction during Q-switched Nd:YAG laser tattoo removal. J Drugs Dermatol. 2015;14:303-306.
- Hibler BP, Rossi AM. A case of delayed anaphylaxis after laser tattoo removal. JAAD Case Rep. 2015;1:80-81.
- Bernstein EF. A widespread allergic reaction to black tattoo ink caused by laser treatment. Lasers Surg Med. 2015;47:180-182.
- Meesters AA, De Rie MA, Wolkerstorfer A. Generalized eczematous reaction after fractional carbon dioxide laser therapy for tattoo allergy. J Cosmet Laser Ther. 2016;18:456-458.
Establishing Financial Literacy: What Every Resident Needs to Know
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
The average debt of graduating medical students today is $190,000, which has increased from $32,000 since 1986 (or the equivalent of $70,000 in 2017 dollars when adjusted for inflation).1 This fact is especially disconcerting given that medical trainees and professionals are not known for being financially sophisticated, and rising levels of high-interest educational debt, increasing years of training, and stagnant or decreasing physician salaries make this status quo untenable.2 Building foundational financial literacy and establishing good financial practices should start during medical school and residency; these basics are a crucial component of long-term job satisfaction and professional resilience.
One prominent physician finance writer advocates that residents should consider the following 5 big-ticket financial steps: acquire life and disability insurance, open a Roth IRA, engage yearly in some type of financial education, and learn about billing and coding in your specialty.3 These exercises, except life insurance for a resident without dependents, are all nonnegotiable, yet alone are insufficient actions to build a solid financial foundation. The purpose of this article is to address additional steps every resident should take, including establishing a workable budget, learning how and why to calculate net worth yearly, determining what percentage of income to save for retirement and basic investing strategies, and managing student loans.
Establish a Workable Budget
Living on a budget is a form of reality acceptance. It may feel impossible to save or budget on a resident salary, but residents earn approximately the median US household income of $59,039, according to the US Census Bureau from September 2017.4,5 There are many tools that can be used to create a budget and to track monthly expenses. However, the simplest way to budget is to pay yourself first with automatic deductions to retirement and savings accounts as well as automated bill payments. Making a habit of reviewing all expenses at the end of every month allows you to see if expenditures remain aligned to your personal values and to reallocate funds for the upcoming month if they are not.
Calculate Net Worth Yearly
Calculating personal net worth may appear to be a discouraging activity to advocate for residents, as many will have a negative 6-figure net worth. The purpose is two-fold: Firstly, to compel you to become well acquainted with your varying types of debt and their respective interest rates. Secondly, similar to taking serial photographs of vitiligo patients to monitor for improvement, it may be the only thing in a long slow slog that indicates beneficial change is occurring because small daily efforts over time yield surprisingly impressive results and the calculation factors in both debt repayment and contributions to all savings vehicles. An example of a simplified method to calculate net worth is demonstrated in the Table.
Understand Your Retirement Account and Asset Distribution
Contributing to a retirement account should start day 1 of intern year. A simple rule of thumb to estimate how much money you need to save for retirement is to divide how much you expect to spend on a yearly basis by 4%. For example, if you anticipate spending $80,000 per year during retirement, you will need $2 million in savings (0.04×$2,000,000=$80,000). The amount saved depends on the aggressiveness of your financial goals, but it should be a minimum of 10% to 15% of income during residency and at least 20% afterwards. This strategy allows even a resident to save $25,000 to $50,000 over a 4-year period (depending on employer match), which can accrue additional value in the stock market. One advantage of contributing to an employer-based retirement account, which usually is a 403(b) plan for residents, is that it lowers your tax burden for the year because the savings are tax deferred, in contrast to a Roth IRA, which is funded with posttax dollars. Roth accounts often are recommended for residents because contributions are made during a period in which the physician is presumably in the lowest tax bracket, as account earnings and withdrawals from a Roth IRA after 59.5 years of age, when most physicians expect to be in a higher tax bracket, are tax free. Another advantage of contributing to a 403(b) account is that many residency programs offer a match, which provides for an immediate and substantial return on invested money. Because most residents do not have the cash flow to fully fund both a Roth IRA and 403(b) account (2018 contribution limits are $5500 and $18,500, respectively),6,7 one strategy to utilize both is to save enough to the 403(b) to capture the employer match and place whatever additional savings you can afford into the Roth IRA.
Many different investment strategies exist, and a thorough discussion of them is beyond the scope of this article. Simply speaking, there are 4 major asset classes in which to invest: US stocks, foreign stocks, real estate, and bonds. The variation of recommended contributions to each asset is limitless, and every resident should spend time considering the best strategy for his/her goals. One example of a simple effective investing strategy is to utilize index funds, which track the market and therefore rise with the market, as they tend to go up (at least historically, though temporary setbacks occur).8 If you are investing in funds available through your employer-sponsored retirement account, examine the funds you are automatically assigned and their associated fee and expense ratio (ER) disclosures, which are typically available through the online portal. A general rule of thumb is that good funds have ERs of less than 0.5% and bad funds have ERs greater than 1% and additional associated fees. The funds available to you also can be researched on the Morningstar, Inc, website (www.morningstar.com). My institution (University of Texas Dell Medical School, Austin) offers a variety of options with ERs varying from 0.02% to 1.02%. The difference in the costs associated with these funds over decades is notable, and it pays (literally) to understand the nuances. Reallocation of funds usually can be done easily online and are effective within 24 hours.
Student Loans
Although many residents agonize most over management of student loans, the simple solution is do not defer them. Refinancing federal loans with a private company versus enrolling in an income-based repayment program depends on many factors, including whether you have a high-earning spouse, how many dependents you have, and whether you expect to stay in academia and will be eligible for Public Service Loan Forgiveness, among others. Look critically at your situation and likely future employment to decide what is most appropriate for you; doing so can save you thousands of dollars in interest over the course of your residency.
Final Thoughts
To the detriment of residents and the attending physicians they will become, discussing financial matters in medicine remains rare, perhaps because it seems to shift what should be the singular focus of our profession, namely to help the sick, to thoughts of personal gain, which is a false dichotomy. Unquestionably, the physician’s role that supersedes all others is to care for the patient and to honor the oath we all took: “Into whatsoever houses I enter, I will enter to help the sick.” But this commitment should not preclude the mastery of financial concepts that promote personal and professional health and well-being. After all, the joy in work is maximized when you are not enslaved to it.
Your reading assignment, paper revision, or presentation can wait. Making time to understand your current financial health, to build your own financial literacy, and to plan for your future is an important component of a long satisfying career. Start now.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
- Grischkan J, George BP, Chaiyachati K, et al. Distribution of medical education debt by specialty, 2010-2016. JAMA Intern Med. 2017;177:1532-1535.
- Ahmad FA, White AJ, Hiller KM, et al. An assessment of residents’ and fellows’ personal finance literacy: an unmet medical education need. Int J Med Educ. 2017;8:192-204.
- The five big money items you should do as a resident. The White Coat Investor website. https://www.whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident. Published July 7, 2011. Accessed May 14, 2018.
- Income, poverty and health insurance coverage in the United States: 2016. United States Census Bureau website. https://www.census.gov/newsroom/press-releases/2017/income-povery.html. Published September 12, 2017. Accessed May 14, 2018.
- Levy S. Residents salary and debt report 2017. Medscape website. https://www.medscape.com/slideshow/residents-salary-and-debt-report-2017-6008931. Published July 26, 2017. Accessed May 22, 2018.
- Retirement topics - IRA contribution limits. Internal Revenue Service website. https://www.irs.gov/retirement-plans/plan-participant-employee/retirement-topics-ira-contribution-limits. Updated October 20, 2017. Accessed May 22, 2018.
- Retirement plan FAQs regarding 403(b) tax-sheltered annuity plans. Internal Revenue Service website. https://www.irs.gov/retirement-plans/retirement-plans-faqs-regarding-403b-tax-sheltered-annuity-plans#conts. Updated November 14, 2017. Accessed May 22, 2018.
- Collins JL. Stock series. JLCollins website. http://jlcollinsnh.com/stock-series/. Accessed May 14, 2018.
Reflections on providing on-call overnight care for psychiatric patients
A transition is coming. My fourth and final year of residency starts soon – meaning that from July 1, 2018, I’ll never again be on call overnight as a psychiatry resident. July 1 marks the end of 2 years as a PGY2 and PGY3, during which I’ve worked 60 overnight shifts.
In our residency program, the on-call shift is a continuation of daytime duties, and the experience is a formative physician-in-training/quasi-hazing process of care provision for 24 hours straight. Previously, I’ve recounted experiences from my overnight on-call shifts and reflected on the intensity of working with emotionally distressed individuals in the emergency department. I never thought I’d say it, but I will miss working in the middle of the night, particularly in the ED. In the small hours of the morning, the strange aura of hospital existence, and desperation of sickness isn’t washed out by daylight and the inhibitions of business hours.
As part of yearlong monitoring research, I’ve asked my fellow residents at George Washington University, in Washington, to participate in a quality improvement survey. It collects information on the number of patients evaluated overnight while on call and also asks residents to rate their on-call experiences on an “emotional pain scale” with space for a qualitative comment. The emotional pain scale is a simple visual analog scale for pain, with a smiley face representing 0 pain and the sad face with tears representing the 10 out of 10, worst pain imaginable. Initially, the emotional pain scale seemed a lighthearted and somewhat silly way for residents to vent about their on-call experience. A year of data collection later, I consider the emotional pain scale an important acknowledgment to my fellow residents that being on call is physically and emotionally taxing.
At the 2018 American Psychiatric Association meeting in New York, I presented findings from this survey data, examining the quantitative information showing that sheer volume of patients correlates with higher emotional pain scores. But while compiling and analyzing the data for my presentation, I also found myself reading and rereading the comments left by my colleagues about their hardest nights. As I read, I reflected on my own 60 nights on call, and my personal experiences between the highs and lows of emotional pain.
As an homage to the educational and emotional power of being on call, I’d like to share a few vignettes from my years of overnight calls from across the emotional pain scale. (Key demographic details have been omitted to protect patients’ privacy.):
- 0: There is no such thing as 0 emotional pain when asked to stay overnight in the hospital.
- 1: This is a full night of sleep on the uncomfortable bunk beds in the GWU call room. On occasion, I’ve had a night with one consult. Only once in 2 years did I have a night in which not a single consult was called from the ED and all the patients on the psychiatric unit slept as soundly as I did.
- 4: There’s a man I’ve seen in the ED seven times over the last 2 years. That’s more than 10% of my nights on call, so we’re well acquainted – though he has trouble remembering me. He’s an alcoholic, though I know the official diagnosis is alcohol use disorder–severe. His addiction is, indeed, severe; I’ve never seen him sober. Every time, he tells me how his wife is cheating on him, and he’s been depressed since his eldest son was killed in a shooting 10 years ago. He sits under a bridge and drinks liquor until he either goes home or to an ED. I feel for him. Several times, other residents and I have transferred him to a local detoxification unit with discharge to a 30-day drug rehab program. It doesn’t stick. The last time I evaluated him, I sent him home to his wife with a cab voucher. My emotional pain is equal parts pity and frustration over my ineffectual impact on his life. He, and others like him, used to cause me more emotional pain. Eventually, the pain is dulled.
Dr. Jacqueline Posada - 5: The mean pain scale score of a GWU resident on call is 5.13. Analysis of the survey data showed the emotional pain score is correlated with the specific resident on call, and my personal average is 5.04. From the way residents talk about being on call, I expected the mean pain scale to be higher. There is no vignette for the mean score; I think of it as all the unremarkable calls blurred together.
- 8: Emotional pain rises with a fraught clinical scenario. One weeknight, I had to involuntarily commit a young lawyer who was psychotic yet adept at hiding it. The lawyer was brought into the ED by police after his brother in Chicago called them to his apartment. The patient had called the brother while standing on his 10th-floor balcony talking nonsensically about conspiracy theories and why he needed to end his life to save the world. In the ED, the patient denied every single part of the story. When I called the brother for collateral, his distress moved me as a both sibling and psychiatrist. The lawyer denied the story up and down, called his brother a liar and the favorite child, and refused to sign into the psychiatric hospital voluntarily. I felt I had no choice but to place him in an involuntary hold. It was a long and busy night, and I tried to put myself in his position and that of his brother who had called the police. Eight is the emotional pain of involuntarily committing someone whose story isn’t black and white. Eight is the pain of exercising authority and beneficence over patient autonomy.
- 10: I was consulted to evaluate a woman for suicidal ideation whose mother had coded and died in the ED an hour before. When the woman was told of her mother’s death, she crumpled to floor, screaming she wanted to die to join her mother. In the tumult, ED staff thought she was running out of the hospital to jump into traffic. She was held in the ED involuntarily until she could be evaluated for safety by psychiatry – me. When I entered her hospital room, she was quietly weeping, whispering: “I want my mom. I want my mom.” I wanted to cry, too. I sat with her in silence for a few minutes and offered my condolences. Yet, as the consultant, I had a job to do: I needed to complete a risk assessment. My voice caught as I explained that I was here to assess her for suicidal thoughts and plans. She looked at me like I was crazy. I felt crazy. I acknowledged the risk of suicide and her expressed desire to join her mother in death. I asked the questions quickly and gently. She shook her head to all my questions and told me she just wanted to go home. I met her daughters in the waiting room who were caught up in grief over the combination of their grandmother’s death and their mother’s reaction. They seemed certain that their mother had not wanted to die, and we agreed it was a situation of the wrong reaction in the wrong place. The daughters agreed to take her home and watch her all night. This is the only 10 I’ve experienced on the emotional pain scale. I felt shame and confusion as I struggled to reconcile my obligation as a psychiatrist, and my true desire to give that woman a hug and send her home without a battery of questions at perhaps her most vulnerable moment.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at GWU. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
A transition is coming. My fourth and final year of residency starts soon – meaning that from July 1, 2018, I’ll never again be on call overnight as a psychiatry resident. July 1 marks the end of 2 years as a PGY2 and PGY3, during which I’ve worked 60 overnight shifts.
In our residency program, the on-call shift is a continuation of daytime duties, and the experience is a formative physician-in-training/quasi-hazing process of care provision for 24 hours straight. Previously, I’ve recounted experiences from my overnight on-call shifts and reflected on the intensity of working with emotionally distressed individuals in the emergency department. I never thought I’d say it, but I will miss working in the middle of the night, particularly in the ED. In the small hours of the morning, the strange aura of hospital existence, and desperation of sickness isn’t washed out by daylight and the inhibitions of business hours.
As part of yearlong monitoring research, I’ve asked my fellow residents at George Washington University, in Washington, to participate in a quality improvement survey. It collects information on the number of patients evaluated overnight while on call and also asks residents to rate their on-call experiences on an “emotional pain scale” with space for a qualitative comment. The emotional pain scale is a simple visual analog scale for pain, with a smiley face representing 0 pain and the sad face with tears representing the 10 out of 10, worst pain imaginable. Initially, the emotional pain scale seemed a lighthearted and somewhat silly way for residents to vent about their on-call experience. A year of data collection later, I consider the emotional pain scale an important acknowledgment to my fellow residents that being on call is physically and emotionally taxing.
At the 2018 American Psychiatric Association meeting in New York, I presented findings from this survey data, examining the quantitative information showing that sheer volume of patients correlates with higher emotional pain scores. But while compiling and analyzing the data for my presentation, I also found myself reading and rereading the comments left by my colleagues about their hardest nights. As I read, I reflected on my own 60 nights on call, and my personal experiences between the highs and lows of emotional pain.
As an homage to the educational and emotional power of being on call, I’d like to share a few vignettes from my years of overnight calls from across the emotional pain scale. (Key demographic details have been omitted to protect patients’ privacy.):
- 0: There is no such thing as 0 emotional pain when asked to stay overnight in the hospital.
- 1: This is a full night of sleep on the uncomfortable bunk beds in the GWU call room. On occasion, I’ve had a night with one consult. Only once in 2 years did I have a night in which not a single consult was called from the ED and all the patients on the psychiatric unit slept as soundly as I did.
- 4: There’s a man I’ve seen in the ED seven times over the last 2 years. That’s more than 10% of my nights on call, so we’re well acquainted – though he has trouble remembering me. He’s an alcoholic, though I know the official diagnosis is alcohol use disorder–severe. His addiction is, indeed, severe; I’ve never seen him sober. Every time, he tells me how his wife is cheating on him, and he’s been depressed since his eldest son was killed in a shooting 10 years ago. He sits under a bridge and drinks liquor until he either goes home or to an ED. I feel for him. Several times, other residents and I have transferred him to a local detoxification unit with discharge to a 30-day drug rehab program. It doesn’t stick. The last time I evaluated him, I sent him home to his wife with a cab voucher. My emotional pain is equal parts pity and frustration over my ineffectual impact on his life. He, and others like him, used to cause me more emotional pain. Eventually, the pain is dulled.
Dr. Jacqueline Posada - 5: The mean pain scale score of a GWU resident on call is 5.13. Analysis of the survey data showed the emotional pain score is correlated with the specific resident on call, and my personal average is 5.04. From the way residents talk about being on call, I expected the mean pain scale to be higher. There is no vignette for the mean score; I think of it as all the unremarkable calls blurred together.
- 8: Emotional pain rises with a fraught clinical scenario. One weeknight, I had to involuntarily commit a young lawyer who was psychotic yet adept at hiding it. The lawyer was brought into the ED by police after his brother in Chicago called them to his apartment. The patient had called the brother while standing on his 10th-floor balcony talking nonsensically about conspiracy theories and why he needed to end his life to save the world. In the ED, the patient denied every single part of the story. When I called the brother for collateral, his distress moved me as a both sibling and psychiatrist. The lawyer denied the story up and down, called his brother a liar and the favorite child, and refused to sign into the psychiatric hospital voluntarily. I felt I had no choice but to place him in an involuntary hold. It was a long and busy night, and I tried to put myself in his position and that of his brother who had called the police. Eight is the emotional pain of involuntarily committing someone whose story isn’t black and white. Eight is the pain of exercising authority and beneficence over patient autonomy.
- 10: I was consulted to evaluate a woman for suicidal ideation whose mother had coded and died in the ED an hour before. When the woman was told of her mother’s death, she crumpled to floor, screaming she wanted to die to join her mother. In the tumult, ED staff thought she was running out of the hospital to jump into traffic. She was held in the ED involuntarily until she could be evaluated for safety by psychiatry – me. When I entered her hospital room, she was quietly weeping, whispering: “I want my mom. I want my mom.” I wanted to cry, too. I sat with her in silence for a few minutes and offered my condolences. Yet, as the consultant, I had a job to do: I needed to complete a risk assessment. My voice caught as I explained that I was here to assess her for suicidal thoughts and plans. She looked at me like I was crazy. I felt crazy. I acknowledged the risk of suicide and her expressed desire to join her mother in death. I asked the questions quickly and gently. She shook her head to all my questions and told me she just wanted to go home. I met her daughters in the waiting room who were caught up in grief over the combination of their grandmother’s death and their mother’s reaction. They seemed certain that their mother had not wanted to die, and we agreed it was a situation of the wrong reaction in the wrong place. The daughters agreed to take her home and watch her all night. This is the only 10 I’ve experienced on the emotional pain scale. I felt shame and confusion as I struggled to reconcile my obligation as a psychiatrist, and my true desire to give that woman a hug and send her home without a battery of questions at perhaps her most vulnerable moment.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at GWU. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
A transition is coming. My fourth and final year of residency starts soon – meaning that from July 1, 2018, I’ll never again be on call overnight as a psychiatry resident. July 1 marks the end of 2 years as a PGY2 and PGY3, during which I’ve worked 60 overnight shifts.
In our residency program, the on-call shift is a continuation of daytime duties, and the experience is a formative physician-in-training/quasi-hazing process of care provision for 24 hours straight. Previously, I’ve recounted experiences from my overnight on-call shifts and reflected on the intensity of working with emotionally distressed individuals in the emergency department. I never thought I’d say it, but I will miss working in the middle of the night, particularly in the ED. In the small hours of the morning, the strange aura of hospital existence, and desperation of sickness isn’t washed out by daylight and the inhibitions of business hours.
As part of yearlong monitoring research, I’ve asked my fellow residents at George Washington University, in Washington, to participate in a quality improvement survey. It collects information on the number of patients evaluated overnight while on call and also asks residents to rate their on-call experiences on an “emotional pain scale” with space for a qualitative comment. The emotional pain scale is a simple visual analog scale for pain, with a smiley face representing 0 pain and the sad face with tears representing the 10 out of 10, worst pain imaginable. Initially, the emotional pain scale seemed a lighthearted and somewhat silly way for residents to vent about their on-call experience. A year of data collection later, I consider the emotional pain scale an important acknowledgment to my fellow residents that being on call is physically and emotionally taxing.
At the 2018 American Psychiatric Association meeting in New York, I presented findings from this survey data, examining the quantitative information showing that sheer volume of patients correlates with higher emotional pain scores. But while compiling and analyzing the data for my presentation, I also found myself reading and rereading the comments left by my colleagues about their hardest nights. As I read, I reflected on my own 60 nights on call, and my personal experiences between the highs and lows of emotional pain.
As an homage to the educational and emotional power of being on call, I’d like to share a few vignettes from my years of overnight calls from across the emotional pain scale. (Key demographic details have been omitted to protect patients’ privacy.):
- 0: There is no such thing as 0 emotional pain when asked to stay overnight in the hospital.
- 1: This is a full night of sleep on the uncomfortable bunk beds in the GWU call room. On occasion, I’ve had a night with one consult. Only once in 2 years did I have a night in which not a single consult was called from the ED and all the patients on the psychiatric unit slept as soundly as I did.
- 4: There’s a man I’ve seen in the ED seven times over the last 2 years. That’s more than 10% of my nights on call, so we’re well acquainted – though he has trouble remembering me. He’s an alcoholic, though I know the official diagnosis is alcohol use disorder–severe. His addiction is, indeed, severe; I’ve never seen him sober. Every time, he tells me how his wife is cheating on him, and he’s been depressed since his eldest son was killed in a shooting 10 years ago. He sits under a bridge and drinks liquor until he either goes home or to an ED. I feel for him. Several times, other residents and I have transferred him to a local detoxification unit with discharge to a 30-day drug rehab program. It doesn’t stick. The last time I evaluated him, I sent him home to his wife with a cab voucher. My emotional pain is equal parts pity and frustration over my ineffectual impact on his life. He, and others like him, used to cause me more emotional pain. Eventually, the pain is dulled.
Dr. Jacqueline Posada - 5: The mean pain scale score of a GWU resident on call is 5.13. Analysis of the survey data showed the emotional pain score is correlated with the specific resident on call, and my personal average is 5.04. From the way residents talk about being on call, I expected the mean pain scale to be higher. There is no vignette for the mean score; I think of it as all the unremarkable calls blurred together.
- 8: Emotional pain rises with a fraught clinical scenario. One weeknight, I had to involuntarily commit a young lawyer who was psychotic yet adept at hiding it. The lawyer was brought into the ED by police after his brother in Chicago called them to his apartment. The patient had called the brother while standing on his 10th-floor balcony talking nonsensically about conspiracy theories and why he needed to end his life to save the world. In the ED, the patient denied every single part of the story. When I called the brother for collateral, his distress moved me as a both sibling and psychiatrist. The lawyer denied the story up and down, called his brother a liar and the favorite child, and refused to sign into the psychiatric hospital voluntarily. I felt I had no choice but to place him in an involuntary hold. It was a long and busy night, and I tried to put myself in his position and that of his brother who had called the police. Eight is the emotional pain of involuntarily committing someone whose story isn’t black and white. Eight is the pain of exercising authority and beneficence over patient autonomy.
- 10: I was consulted to evaluate a woman for suicidal ideation whose mother had coded and died in the ED an hour before. When the woman was told of her mother’s death, she crumpled to floor, screaming she wanted to die to join her mother. In the tumult, ED staff thought she was running out of the hospital to jump into traffic. She was held in the ED involuntarily until she could be evaluated for safety by psychiatry – me. When I entered her hospital room, she was quietly weeping, whispering: “I want my mom. I want my mom.” I wanted to cry, too. I sat with her in silence for a few minutes and offered my condolences. Yet, as the consultant, I had a job to do: I needed to complete a risk assessment. My voice caught as I explained that I was here to assess her for suicidal thoughts and plans. She looked at me like I was crazy. I felt crazy. I acknowledged the risk of suicide and her expressed desire to join her mother in death. I asked the questions quickly and gently. She shook her head to all my questions and told me she just wanted to go home. I met her daughters in the waiting room who were caught up in grief over the combination of their grandmother’s death and their mother’s reaction. They seemed certain that their mother had not wanted to die, and we agreed it was a situation of the wrong reaction in the wrong place. The daughters agreed to take her home and watch her all night. This is the only 10 I’ve experienced on the emotional pain scale. I felt shame and confusion as I struggled to reconcile my obligation as a psychiatrist, and my true desire to give that woman a hug and send her home without a battery of questions at perhaps her most vulnerable moment.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at GWU. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
Climate Change and Skin Disease
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
The term climate refers to the average weather conditions of a specific geographic location measured over several decades.1 While a certain degree of variation in the Earth’s climate is expected, a persistent warming or cooling trend is not. The factors driving the Earth’s warming remain difficult to prove.2 We know the Earth previously has undergone dramatic climate changes and that natural factors driving these changes are varied (eg, the relationship between the Earth and the Sun, volcanic eruptions, solar irradiance).1,3 These factors ideally change over protracted periods of time in a way that allows organisms to adapt to new environments.
Anthropogenic climate change refers to human-caused climate change. This is thought to be a major driving factor in the Earth’s recent warming trend, partly due to the rapidity of warming in recent years.3 According to climate scientists, the Earth’s temperature has risen 4°C to 7°C over the past 5000 years, but it has risen 0.7°C in just the past 100 years alone.4 Greenhouse gases such as carbon dioxide are emitted by various natural processes and human activities and play a central role in current warming because they trap solar heat and increase ambient temperature.3
In a recent edition of the commonly cited textbook Dermatology, Bolognia et al5 referenced climate change only once in a figure legend regarding the expansion of dengue fever in the Americas. However, climate change may have the potential to cause outright skin disease epidemics worldwide, and the Climate Change Committee of the International Society of Dermatology has called upon dermatologists across the globe to help raise awareness of this issue.6
Much of the literature regarding the effects of climate change on human health focuses on insect-borne diseases, but over the past decade other areas of impact also have been investigated, such as increases in airborne diseases, zoonoses, newly endemic saprophytic and dimorphic fungal infections, fecal-oral diseases, and severe allergic disease.7,8 It is postulated that climate change leads to region-specific increases in human disease because it creates newly favorable habitats for infectious agents, their vectors. and their reservoirs, allowing expansion of their ranges and access to immunologically naïve populations.9 Furthermore, extreme weather events such as heat waves, hurricanes, and flooding, which are expected to increase in frequency as a result of climate change, have all been linked to infectious disease outbreaks.10
Lyme Disease
In the past 20 years, Lyme disease incidence has tripled in the United States.11 It has been hypothesized that the increase may be occurring as a result of the expanding geographic distribution of the Ixodes tick and its mammalian hosts (eg, white-tailed deer) under the influence of climate change.12 Lyme disease is a multisystem disease affecting the skin, joints, heart, and nervous system. Its most characteristic manifestation is cutaneous in the form of erythema migrans. Dermatologists may be called upon to play an increasingly important role in early detection and treatment of this potentially chronic and debilitating condition.
Arboviruses
Arboviruses are transmitted by arthropods and are an important category of climate change–related diseases due to the expansion of the mosquito habitat worldwide. The vectorial capacity for the transmission of dengue fever has increased worldwide by 9.4% via Aedes aegypti and 11.1% via Aedes albopictus since 1950.13 Dengue fever, also known as breakbone fever, presents with intense joint pain, fevers, headaches, and a transient morbilliform rash that desquamates with defervescence and in some cases will incite hemorrhagic skin lesions.14 Dengue fever previously was considered to be a tropical disease but locally acquired cases have been reported in the United States, including Texas, Hawaii, and Florida.15,16
Reports of local transmission of chikungunya, another arbovirus transmitted by A albopictus and A aegypti mosquitoes in Florida, the US Virgin Islands, and Puerto Rico, began in 2014.17 A higher prevalence of these diseases within the United States also may be related to increased globalization, with US travelers returning from endemic regions with infections. Prior to 2014, transmission occurred in traditional endemic regions, primarily in Asia, Africa, or island nations in the Indian Ocean. Like dengue fever, chikungunya causes high fevers, cutaneous manifestations (eg, urticarial papules, morbilliform eruption, hypermelanosis, intertriginous lesions, lymphedema),17 and intense joint pain. Unlike dengue fever, however, joint involvement can be chronic, erosive, and debilitating.
Lastly, New World leishmaniasis, an arboviral disease characterized by mucocutaneous ulcers and transmitted by phlebotomine sand flies, has been acquired locally in Oklahoma and Texas when it was previously considered to be endemic to Mexico and Central and South America.14,18 The habitats of New World Leishmania species are expected to expand northward, with an ecological niche model predicting that they reach southern Canada by the year 2080 due to the expanding habitats of sand fly and rodent vectors.19
Fungal Infections
In the Pacific Northwest, there have been reports of newly endemic Cryptococcus gattii and Coccidioides immitis, both of which previously had been confined to the southwestern United States.8 Endemic ranges of these mycotic pathogens may be expanding for a variety of reasons, with climate change creating new regions conducive to the colonization of these species.8,20,21Coccidioides immitis is a soil-dwelling fungus that usually presents with primary pulmonary disease that can disseminate acutely or even months later. Prompt recognition of disseminated disease may allow life-saving therapy to be initiated. Cryptococcus gattii is a fungus with multiple niches, including oil, trees, and birds.20 This fungus also is acquired via inhalation, with dissemination occurring most commonly in immunosuppressed patients to the central nervous system, bone, and skin. Primary or secondary infection with both of these fungi may present with cutaneous manifestations presenting as polymorphous lesions, including umbilicated or ulcerated papules, indurated nodules, and acneiform pustules.
Final Thoughts
Awareness of the shifting habitats of microorganisms and vectors locally is important in order for clinicians to make correct diagnoses in a timely fashion. Regional or endemic diseases are presenting outside their traditional boundaries due to changing habitats of microbes and vectors and may be easily overlooked, resulting in a delayed diagnosis. Being prepared to diagnose diseases with increasing incidence secondary to climate change and discussing this with patients is an important physician obligation, but it is not the only one. We cannot effectively advocate for the health of patients and the community while ignoring the destruction of the environment. Our additional responsibility is straightforward—being advocates for good stewardship of the Earth’s resources now on both a personal and a policy level.22
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.
- Climate Central. Global Weirdness: Severe Storms, Deadly Heat Waves, Relentless Drought, Rising Seas, and the Weather of the Future. New York, NY: Pantheon Books; 2012.
- Cook J, Nuccitelli D, Green SA, et al. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ Res Lett. 2013;8:024024.
- A blanket around the Earth. NASA Climate website. https://climate.nasa.gov/causes/. Accessed February 5, 2018.
- How is today’s warming different from the past? NASA Earth Observatory website. https://earthobservatory.nasa.gov/Features/GlobalWarming/page3.php. Accessed February 5, 2018.
- Mancini AJ, Shani-Adir A, Sidbury R. Other viral diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2017:1425-1446.
- Andersen LK, Davis MDP. A wake-up call to dermatologists—climate change affects the skin. Int J Dermatol. 2017;56:E198-E199.
- Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives [published online March 23, 2017]. Environ Int. 2017;103:99-108.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging fungal infections in the Pacific Northwest: the unrecognized burden and geographic range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016;4. doi:10.1128/microbiolspec.EI10-0016-2016.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946-1955.
- McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543-547.
- Lyme disease graphs. CDC website. https://www.cdc.gov/lyme/stats/graphs.html. Updated November 1, 2017. Accessed April 12, 2018.
- Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 2017;17:619-629.
- Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health [published online October 30, 2017]. Lancet. doi:10.1016/S0140-6736(17)32464-9.
- Nawas ZY, Tong Y, Kollipara R, et al. Emerging infectious diseases with cutaneous manifestations: viral and bacterial infections. J Am Acad Dermatol. 2016;75:1-16.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Dengue. CDC website. https://www.cdc.gov/dengue/epidemiology/index.html. Updated June 9, 2014. Accessed April 3, 2018.
- Chikungunya virus in the United States. CDC website. https://www.cdc.gov/chikungunya/geo/united-states.html. Updated October 30, 2017. Accessed April 4, 2018.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-61.
- González C, Wang O, Strutz SE, et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:E585.
- Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel). 2015;2. doi: 10.3390/jof2010001.
- Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56:847-850.
- Rosenbach M. Climate change & dermatology: what can you do? Paper presented at: American Academy of Dermatology Annual Meeting; March 3-7, 2017; Orlando, FL.
What Do You Want to Be When You Grow Up? Pearls for Postresidency Planning
Dermatology residency training can feel endless at the outset; an arduous intern year followed by 3 years of specialized training. However, I have realized that, within residency, time moves quickly. As I look ahead to postresidency life, I realize that residents are all facing the same question: What do you want to be when you grow up?
You may think you have answered that question already; however, there are many different careers within the field of dermatology and no amount of studying or reading will help you choose the right one. In an attempt to make sense of these choices, I have spoken to many recent dermatology graduates over the last several months to get a sense of how they made their postresidency decisions, and I want to share their pearls.
Pearl: Explore Fellowship Opportunities Early
The first decision is whether or not to pursue a fellowship after residency. There currently are 2 Accreditation Council for Graduate Medical Education–approved fellowships after dermatology residency: dermatopathology and micrographic surgery. Pediatric dermatology is another board-certified fellowship. A list of these training programs and the requirements can be found on the American Board of Dermatology website (www.abderm.org). There also are several nonaccredited fellowships including pediatrics, cosmetics, complex medical dermatology, cutaneous oncology, and rheumatology.
Even if you are not completely committed to pursuing a fellowship, it is beneficial to explore any fellowship options early in residency. Spend extra time in any field you are considering for fellowship and consider research in the field. If there is a fellowship position at your institution, try to rotate there early in residency. Rotations at other institutions can demonstrate your interest and enthusiasm while also helping you to network within your chosen subspecialty. Several of the dermatology interest groups even sponsor rotations at outside institutions, if extra funding is needed. If recent graduates from your program have matched in fellowship, it is always a good idea to reach out to them to get program-specific advice. It takes a lot of time, confidence, and persistence to organize the opportunities that will help you maximize your fellowship potential, but it is well worth the effort.
Fellowships can occur through an official “match,” similar to residency, or can be accepted on a rolling basis. For example, many dermatopathology fellowships can begin accepting applications as early as the summer between the first and second year of residency (www.abderm.org). It is important to get this information early so that you do not miss any application deadlines.
Pearl: Prioritize Where You Want to Practice
If you have decided that fellowship is not for you, then it is time to apply for your first job as a physician. There are several big factors that help narrow the search. It is best to start the search early to allow yourself time and different options. According to the 2016 American Academy of Dermatology database, there currently are approximately 3.4 dermatologists per 100,000 Americans; however, they are unevenly distributed throughout the country. In this study, the researchers found the highest density of dermatologists on the Upper East Side of Manhattan (41.8 per 100,000 dermatologists) compared to Swainsboro, Georgia (0.45 per 100,000 dermatologists).1
With more competition for jobs in areas with a higher concentration of dermatologists, compensation often is lower. There also are many personal factors that contribute to where you want to live and work, and if you prioritize them, it will lead to greater overall satisfaction in postresidency life.
Another large factor to consider is private practice versus academic dermatology. Academic dermatology can provide opportunities for research as well as the opportunity to work with students and residents. As part of a larger hospital system, there often is the opportunity for benefits, such as 401(k) matching, that might be less accessible in small practices.
Pearl: Get Recruiter Recommendations From Your Peers
There are many recruiting services that can help put you in touch with practices that are hiring. These services can be helpful but also can be overwhelming at times, with many emails and telephone calls. In my experience, recent graduates had mixed feelings about recruiting services. Those who had been the happiest with their recruiting experience had often gotten the name of a specific recruiter from someone else in their program who had a positive experience. Mentors at your training institution or beyond also can be a good source of information for job opportunities. It can be helpful to get involved early in the various dermatologic societies and network at academic conferences throughout your training.
Pearl: Talk to Partners and Nonpartners About the Practice’s Philosophy
When picking a private practice for your first job, make sure you get a sense of the philosophy of the practice, including the partners’ goals for the office, the patient population, and the dynamic of the office staff. If there is a cosmetic component, it is important to know what devices are available and which products are sold. It is important to talk to nonpartners at a practice and get a sense of their satisfaction. If you sign the employment contract, you will be in their shoes soon!
Pearl: Have an Attorney Review Your Contract
There are many important topics in your employment contract. After years of medical school loans and resident salary, it is easy to focus only on compensation. However, pay attention to the other aspects of reimbursement including bonuses, benefits, noncompete clauses, and call schedules. Also consider the termination policies. The general advice I have received is to have a lawyer look at your contract. Although it may be tempting to skip the lawyer’s fee and review it yourself, you may actually end up negotiating a contract that benefits you more in the long-run or avoid signing a contract that will limit you.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
Dermatology residency training can feel endless at the outset; an arduous intern year followed by 3 years of specialized training. However, I have realized that, within residency, time moves quickly. As I look ahead to postresidency life, I realize that residents are all facing the same question: What do you want to be when you grow up?
You may think you have answered that question already; however, there are many different careers within the field of dermatology and no amount of studying or reading will help you choose the right one. In an attempt to make sense of these choices, I have spoken to many recent dermatology graduates over the last several months to get a sense of how they made their postresidency decisions, and I want to share their pearls.
Pearl: Explore Fellowship Opportunities Early
The first decision is whether or not to pursue a fellowship after residency. There currently are 2 Accreditation Council for Graduate Medical Education–approved fellowships after dermatology residency: dermatopathology and micrographic surgery. Pediatric dermatology is another board-certified fellowship. A list of these training programs and the requirements can be found on the American Board of Dermatology website (www.abderm.org). There also are several nonaccredited fellowships including pediatrics, cosmetics, complex medical dermatology, cutaneous oncology, and rheumatology.
Even if you are not completely committed to pursuing a fellowship, it is beneficial to explore any fellowship options early in residency. Spend extra time in any field you are considering for fellowship and consider research in the field. If there is a fellowship position at your institution, try to rotate there early in residency. Rotations at other institutions can demonstrate your interest and enthusiasm while also helping you to network within your chosen subspecialty. Several of the dermatology interest groups even sponsor rotations at outside institutions, if extra funding is needed. If recent graduates from your program have matched in fellowship, it is always a good idea to reach out to them to get program-specific advice. It takes a lot of time, confidence, and persistence to organize the opportunities that will help you maximize your fellowship potential, but it is well worth the effort.
Fellowships can occur through an official “match,” similar to residency, or can be accepted on a rolling basis. For example, many dermatopathology fellowships can begin accepting applications as early as the summer between the first and second year of residency (www.abderm.org). It is important to get this information early so that you do not miss any application deadlines.
Pearl: Prioritize Where You Want to Practice
If you have decided that fellowship is not for you, then it is time to apply for your first job as a physician. There are several big factors that help narrow the search. It is best to start the search early to allow yourself time and different options. According to the 2016 American Academy of Dermatology database, there currently are approximately 3.4 dermatologists per 100,000 Americans; however, they are unevenly distributed throughout the country. In this study, the researchers found the highest density of dermatologists on the Upper East Side of Manhattan (41.8 per 100,000 dermatologists) compared to Swainsboro, Georgia (0.45 per 100,000 dermatologists).1
With more competition for jobs in areas with a higher concentration of dermatologists, compensation often is lower. There also are many personal factors that contribute to where you want to live and work, and if you prioritize them, it will lead to greater overall satisfaction in postresidency life.
Another large factor to consider is private practice versus academic dermatology. Academic dermatology can provide opportunities for research as well as the opportunity to work with students and residents. As part of a larger hospital system, there often is the opportunity for benefits, such as 401(k) matching, that might be less accessible in small practices.
Pearl: Get Recruiter Recommendations From Your Peers
There are many recruiting services that can help put you in touch with practices that are hiring. These services can be helpful but also can be overwhelming at times, with many emails and telephone calls. In my experience, recent graduates had mixed feelings about recruiting services. Those who had been the happiest with their recruiting experience had often gotten the name of a specific recruiter from someone else in their program who had a positive experience. Mentors at your training institution or beyond also can be a good source of information for job opportunities. It can be helpful to get involved early in the various dermatologic societies and network at academic conferences throughout your training.
Pearl: Talk to Partners and Nonpartners About the Practice’s Philosophy
When picking a private practice for your first job, make sure you get a sense of the philosophy of the practice, including the partners’ goals for the office, the patient population, and the dynamic of the office staff. If there is a cosmetic component, it is important to know what devices are available and which products are sold. It is important to talk to nonpartners at a practice and get a sense of their satisfaction. If you sign the employment contract, you will be in their shoes soon!
Pearl: Have an Attorney Review Your Contract
There are many important topics in your employment contract. After years of medical school loans and resident salary, it is easy to focus only on compensation. However, pay attention to the other aspects of reimbursement including bonuses, benefits, noncompete clauses, and call schedules. Also consider the termination policies. The general advice I have received is to have a lawyer look at your contract. Although it may be tempting to skip the lawyer’s fee and review it yourself, you may actually end up negotiating a contract that benefits you more in the long-run or avoid signing a contract that will limit you.
Dermatology residency training can feel endless at the outset; an arduous intern year followed by 3 years of specialized training. However, I have realized that, within residency, time moves quickly. As I look ahead to postresidency life, I realize that residents are all facing the same question: What do you want to be when you grow up?
You may think you have answered that question already; however, there are many different careers within the field of dermatology and no amount of studying or reading will help you choose the right one. In an attempt to make sense of these choices, I have spoken to many recent dermatology graduates over the last several months to get a sense of how they made their postresidency decisions, and I want to share their pearls.
Pearl: Explore Fellowship Opportunities Early
The first decision is whether or not to pursue a fellowship after residency. There currently are 2 Accreditation Council for Graduate Medical Education–approved fellowships after dermatology residency: dermatopathology and micrographic surgery. Pediatric dermatology is another board-certified fellowship. A list of these training programs and the requirements can be found on the American Board of Dermatology website (www.abderm.org). There also are several nonaccredited fellowships including pediatrics, cosmetics, complex medical dermatology, cutaneous oncology, and rheumatology.
Even if you are not completely committed to pursuing a fellowship, it is beneficial to explore any fellowship options early in residency. Spend extra time in any field you are considering for fellowship and consider research in the field. If there is a fellowship position at your institution, try to rotate there early in residency. Rotations at other institutions can demonstrate your interest and enthusiasm while also helping you to network within your chosen subspecialty. Several of the dermatology interest groups even sponsor rotations at outside institutions, if extra funding is needed. If recent graduates from your program have matched in fellowship, it is always a good idea to reach out to them to get program-specific advice. It takes a lot of time, confidence, and persistence to organize the opportunities that will help you maximize your fellowship potential, but it is well worth the effort.
Fellowships can occur through an official “match,” similar to residency, or can be accepted on a rolling basis. For example, many dermatopathology fellowships can begin accepting applications as early as the summer between the first and second year of residency (www.abderm.org). It is important to get this information early so that you do not miss any application deadlines.
Pearl: Prioritize Where You Want to Practice
If you have decided that fellowship is not for you, then it is time to apply for your first job as a physician. There are several big factors that help narrow the search. It is best to start the search early to allow yourself time and different options. According to the 2016 American Academy of Dermatology database, there currently are approximately 3.4 dermatologists per 100,000 Americans; however, they are unevenly distributed throughout the country. In this study, the researchers found the highest density of dermatologists on the Upper East Side of Manhattan (41.8 per 100,000 dermatologists) compared to Swainsboro, Georgia (0.45 per 100,000 dermatologists).1
With more competition for jobs in areas with a higher concentration of dermatologists, compensation often is lower. There also are many personal factors that contribute to where you want to live and work, and if you prioritize them, it will lead to greater overall satisfaction in postresidency life.
Another large factor to consider is private practice versus academic dermatology. Academic dermatology can provide opportunities for research as well as the opportunity to work with students and residents. As part of a larger hospital system, there often is the opportunity for benefits, such as 401(k) matching, that might be less accessible in small practices.
Pearl: Get Recruiter Recommendations From Your Peers
There are many recruiting services that can help put you in touch with practices that are hiring. These services can be helpful but also can be overwhelming at times, with many emails and telephone calls. In my experience, recent graduates had mixed feelings about recruiting services. Those who had been the happiest with their recruiting experience had often gotten the name of a specific recruiter from someone else in their program who had a positive experience. Mentors at your training institution or beyond also can be a good source of information for job opportunities. It can be helpful to get involved early in the various dermatologic societies and network at academic conferences throughout your training.
Pearl: Talk to Partners and Nonpartners About the Practice’s Philosophy
When picking a private practice for your first job, make sure you get a sense of the philosophy of the practice, including the partners’ goals for the office, the patient population, and the dynamic of the office staff. If there is a cosmetic component, it is important to know what devices are available and which products are sold. It is important to talk to nonpartners at a practice and get a sense of their satisfaction. If you sign the employment contract, you will be in their shoes soon!
Pearl: Have an Attorney Review Your Contract
There are many important topics in your employment contract. After years of medical school loans and resident salary, it is easy to focus only on compensation. However, pay attention to the other aspects of reimbursement including bonuses, benefits, noncompete clauses, and call schedules. Also consider the termination policies. The general advice I have received is to have a lawyer look at your contract. Although it may be tempting to skip the lawyer’s fee and review it yourself, you may actually end up negotiating a contract that benefits you more in the long-run or avoid signing a contract that will limit you.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
A trainee’s path to fighting addiction
When I came to this country, even before my current residency, I launched my addiction psychiatry career by researching nicotine addiction in schizophrenia patients. Those early experiences gave me a greater understanding of the health concerns and life experiences of people with addictions – and those more likely to develop them.
So imagine my excitement when I first became acquainted with the American Academy of Addiction Psychiatry (AAAP). I first learned about the AAAP, its mission, and activities at the 2017 American Psychiatric Association’s annual meeting in San Diego.
Getting in and involved
After returning to New York, I took those next steps and joined up, which opened the AAAP gates so I could receive its newsletters and submission calls, gain access to resources such as The American Journal on Addictions, survey the various joinable task forces, as well as discover who might be available to me as a mentor as part of the AAAP’s mentor-mentee program.
Sometime during my third-year residency training, I received a member-email advertising the AAAP 28th Annual Meeting and Scientific Symposium, and soon after that, received another email calling for research submissions to be presented there, as well as an invitation to apply for a trainee travel scholarship that would defray the cost for and allow its fellows to attend the meeting in San Diego. That alone was enticing enough to apply. But even more enticing was the opportunity to showcase the addiction work I had been doing during my residency, as well as to meet other members at various levels of the AAAP to determine whether I wanted to become more involved.
Pursuing experiences
I did not think twice about applying for the poster presentation and the travel scholarship. The AAAP’s online application forms for both were easy to understand and very well structured, which greatly helped me with filling out and formatting my applications. Taking the initiative toward even these first AAAP offerings brought more positive echoes. I was thrilled when the poster I proposed was accepted, mostly because it would give me the chance to present my recent addiction psych work from a higher platform. A few weeks later, I was thrilled again when I received an AAAP email congratulating me on being awarded the San Diego 28th annual meeting travel scholarship, which would waive the annual membership and conference registration fees, in addition to defraying my travel costs. Pacific breezes, here I come. And there I went. (Thanks to my extremely supportive training director, who first nominated me for the award.)
On the ground at 2017 AAAP
The 28th AAAP annual meeting opened on a balmy December Thursday, and that’s the day I arrived. I attended many addiction workshops and symposiums, which featured premier figures in addiction psychiatry. Of the numerous trainee-specific events I attended, the most informative was the “Fellowship Forum: Exploring the Field of Addiction Psychiatry.” At this forum, I learned the true benefits of doing an addiction psychiatry fellowship, while meeting many of the fellowship program directors of top institutions. Having them all under one “roof” made it easy to compare and contrast the specific training they offered.
Then came what were, for me, major highlights of the AAAP 2017. After I delivered my poster presentation and shared my research, I was able to receive very close, constructive feedback from the field’s most experienced professionals. And, finally, I met my AAAP mentors face to face: Dr. Amy Yule of Harvard Medical School, Boston; Dr. Thomas Penders, of East Carolina University, Greenville, N.C.; and Dr. Cornel Stanciu of Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
One AAAP trainee’s takeaways
All AAAP trainees, fellows, presenters leave the meeting with their own conclusions, but my biggest takeaways were:
- Regarding barriers to buprenorphine, emerging research supports similar efficacy for long-acting injectable naltrexone.
- Various protocols for rapid implementation of naltrexone are being used, and these allow for smoother transition and shorter “washout” periods.
- We should not overlook the effects of tobacco use in our patient population – and should address it aggressively, regardless of psychiatric comorbidities.
- The cannabinoid CBD receptors that exist on the dopamine pathway strengthen and complicate their relationship with psychosis.
- , especially in rural and remote settings. The body of evidence supporting its efficacy is expanding.
- Synthetic cannabinoids are prevalent, and toxidromes exist – yet, trainees are not current on these.
The challenges facing those of us dedicated to fighting addiction have never been greater. I would urge more trainees and psychiatrists to join the AAAP in light of the opioid crisis and the potential fallout tied to marijuana legalization. I am grateful to have the opportunity to join my colleagues in this fight. Becoming part of the AAAP has led to a highly rewarding, career-enriching experience.
This article was updated 1/17/17.
Dr. Ahmed is a third-year resident in the department of psychiatry at Nassau University Medical Center, East Meadow, New York. Besides addiction psychiatry, his interests include public social psychiatry, health care policy, health disparities, and mental health stigma. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
When I came to this country, even before my current residency, I launched my addiction psychiatry career by researching nicotine addiction in schizophrenia patients. Those early experiences gave me a greater understanding of the health concerns and life experiences of people with addictions – and those more likely to develop them.
So imagine my excitement when I first became acquainted with the American Academy of Addiction Psychiatry (AAAP). I first learned about the AAAP, its mission, and activities at the 2017 American Psychiatric Association’s annual meeting in San Diego.
Getting in and involved
After returning to New York, I took those next steps and joined up, which opened the AAAP gates so I could receive its newsletters and submission calls, gain access to resources such as The American Journal on Addictions, survey the various joinable task forces, as well as discover who might be available to me as a mentor as part of the AAAP’s mentor-mentee program.
Sometime during my third-year residency training, I received a member-email advertising the AAAP 28th Annual Meeting and Scientific Symposium, and soon after that, received another email calling for research submissions to be presented there, as well as an invitation to apply for a trainee travel scholarship that would defray the cost for and allow its fellows to attend the meeting in San Diego. That alone was enticing enough to apply. But even more enticing was the opportunity to showcase the addiction work I had been doing during my residency, as well as to meet other members at various levels of the AAAP to determine whether I wanted to become more involved.
Pursuing experiences
I did not think twice about applying for the poster presentation and the travel scholarship. The AAAP’s online application forms for both were easy to understand and very well structured, which greatly helped me with filling out and formatting my applications. Taking the initiative toward even these first AAAP offerings brought more positive echoes. I was thrilled when the poster I proposed was accepted, mostly because it would give me the chance to present my recent addiction psych work from a higher platform. A few weeks later, I was thrilled again when I received an AAAP email congratulating me on being awarded the San Diego 28th annual meeting travel scholarship, which would waive the annual membership and conference registration fees, in addition to defraying my travel costs. Pacific breezes, here I come. And there I went. (Thanks to my extremely supportive training director, who first nominated me for the award.)
On the ground at 2017 AAAP
The 28th AAAP annual meeting opened on a balmy December Thursday, and that’s the day I arrived. I attended many addiction workshops and symposiums, which featured premier figures in addiction psychiatry. Of the numerous trainee-specific events I attended, the most informative was the “Fellowship Forum: Exploring the Field of Addiction Psychiatry.” At this forum, I learned the true benefits of doing an addiction psychiatry fellowship, while meeting many of the fellowship program directors of top institutions. Having them all under one “roof” made it easy to compare and contrast the specific training they offered.
Then came what were, for me, major highlights of the AAAP 2017. After I delivered my poster presentation and shared my research, I was able to receive very close, constructive feedback from the field’s most experienced professionals. And, finally, I met my AAAP mentors face to face: Dr. Amy Yule of Harvard Medical School, Boston; Dr. Thomas Penders, of East Carolina University, Greenville, N.C.; and Dr. Cornel Stanciu of Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
One AAAP trainee’s takeaways
All AAAP trainees, fellows, presenters leave the meeting with their own conclusions, but my biggest takeaways were:
- Regarding barriers to buprenorphine, emerging research supports similar efficacy for long-acting injectable naltrexone.
- Various protocols for rapid implementation of naltrexone are being used, and these allow for smoother transition and shorter “washout” periods.
- We should not overlook the effects of tobacco use in our patient population – and should address it aggressively, regardless of psychiatric comorbidities.
- The cannabinoid CBD receptors that exist on the dopamine pathway strengthen and complicate their relationship with psychosis.
- , especially in rural and remote settings. The body of evidence supporting its efficacy is expanding.
- Synthetic cannabinoids are prevalent, and toxidromes exist – yet, trainees are not current on these.
The challenges facing those of us dedicated to fighting addiction have never been greater. I would urge more trainees and psychiatrists to join the AAAP in light of the opioid crisis and the potential fallout tied to marijuana legalization. I am grateful to have the opportunity to join my colleagues in this fight. Becoming part of the AAAP has led to a highly rewarding, career-enriching experience.
This article was updated 1/17/17.
Dr. Ahmed is a third-year resident in the department of psychiatry at Nassau University Medical Center, East Meadow, New York. Besides addiction psychiatry, his interests include public social psychiatry, health care policy, health disparities, and mental health stigma. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
When I came to this country, even before my current residency, I launched my addiction psychiatry career by researching nicotine addiction in schizophrenia patients. Those early experiences gave me a greater understanding of the health concerns and life experiences of people with addictions – and those more likely to develop them.
So imagine my excitement when I first became acquainted with the American Academy of Addiction Psychiatry (AAAP). I first learned about the AAAP, its mission, and activities at the 2017 American Psychiatric Association’s annual meeting in San Diego.
Getting in and involved
After returning to New York, I took those next steps and joined up, which opened the AAAP gates so I could receive its newsletters and submission calls, gain access to resources such as The American Journal on Addictions, survey the various joinable task forces, as well as discover who might be available to me as a mentor as part of the AAAP’s mentor-mentee program.
Sometime during my third-year residency training, I received a member-email advertising the AAAP 28th Annual Meeting and Scientific Symposium, and soon after that, received another email calling for research submissions to be presented there, as well as an invitation to apply for a trainee travel scholarship that would defray the cost for and allow its fellows to attend the meeting in San Diego. That alone was enticing enough to apply. But even more enticing was the opportunity to showcase the addiction work I had been doing during my residency, as well as to meet other members at various levels of the AAAP to determine whether I wanted to become more involved.
Pursuing experiences
I did not think twice about applying for the poster presentation and the travel scholarship. The AAAP’s online application forms for both were easy to understand and very well structured, which greatly helped me with filling out and formatting my applications. Taking the initiative toward even these first AAAP offerings brought more positive echoes. I was thrilled when the poster I proposed was accepted, mostly because it would give me the chance to present my recent addiction psych work from a higher platform. A few weeks later, I was thrilled again when I received an AAAP email congratulating me on being awarded the San Diego 28th annual meeting travel scholarship, which would waive the annual membership and conference registration fees, in addition to defraying my travel costs. Pacific breezes, here I come. And there I went. (Thanks to my extremely supportive training director, who first nominated me for the award.)
On the ground at 2017 AAAP
The 28th AAAP annual meeting opened on a balmy December Thursday, and that’s the day I arrived. I attended many addiction workshops and symposiums, which featured premier figures in addiction psychiatry. Of the numerous trainee-specific events I attended, the most informative was the “Fellowship Forum: Exploring the Field of Addiction Psychiatry.” At this forum, I learned the true benefits of doing an addiction psychiatry fellowship, while meeting many of the fellowship program directors of top institutions. Having them all under one “roof” made it easy to compare and contrast the specific training they offered.
Then came what were, for me, major highlights of the AAAP 2017. After I delivered my poster presentation and shared my research, I was able to receive very close, constructive feedback from the field’s most experienced professionals. And, finally, I met my AAAP mentors face to face: Dr. Amy Yule of Harvard Medical School, Boston; Dr. Thomas Penders, of East Carolina University, Greenville, N.C.; and Dr. Cornel Stanciu of Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
One AAAP trainee’s takeaways
All AAAP trainees, fellows, presenters leave the meeting with their own conclusions, but my biggest takeaways were:
- Regarding barriers to buprenorphine, emerging research supports similar efficacy for long-acting injectable naltrexone.
- Various protocols for rapid implementation of naltrexone are being used, and these allow for smoother transition and shorter “washout” periods.
- We should not overlook the effects of tobacco use in our patient population – and should address it aggressively, regardless of psychiatric comorbidities.
- The cannabinoid CBD receptors that exist on the dopamine pathway strengthen and complicate their relationship with psychosis.
- , especially in rural and remote settings. The body of evidence supporting its efficacy is expanding.
- Synthetic cannabinoids are prevalent, and toxidromes exist – yet, trainees are not current on these.
The challenges facing those of us dedicated to fighting addiction have never been greater. I would urge more trainees and psychiatrists to join the AAAP in light of the opioid crisis and the potential fallout tied to marijuana legalization. I am grateful to have the opportunity to join my colleagues in this fight. Becoming part of the AAAP has led to a highly rewarding, career-enriching experience.
This article was updated 1/17/17.
Dr. Ahmed is a third-year resident in the department of psychiatry at Nassau University Medical Center, East Meadow, New York. Besides addiction psychiatry, his interests include public social psychiatry, health care policy, health disparities, and mental health stigma. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
Pediatric Leg Ulcers: Going Out on a Limb for the Diagnosis
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.