User login
Vesiculobullous and Pustular Diseases in Newborns
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
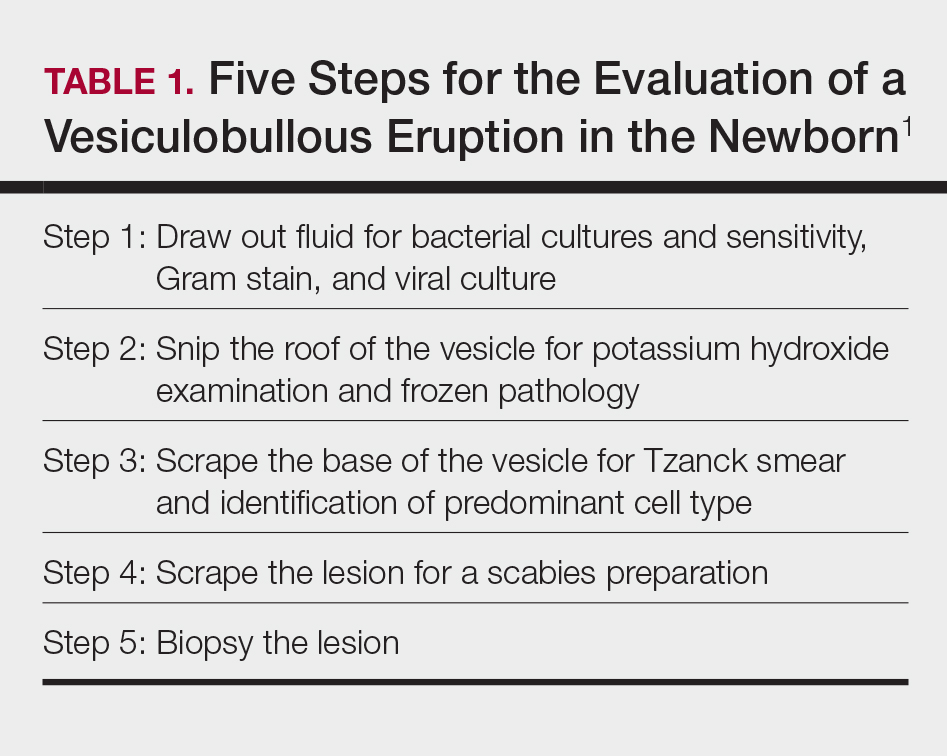
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
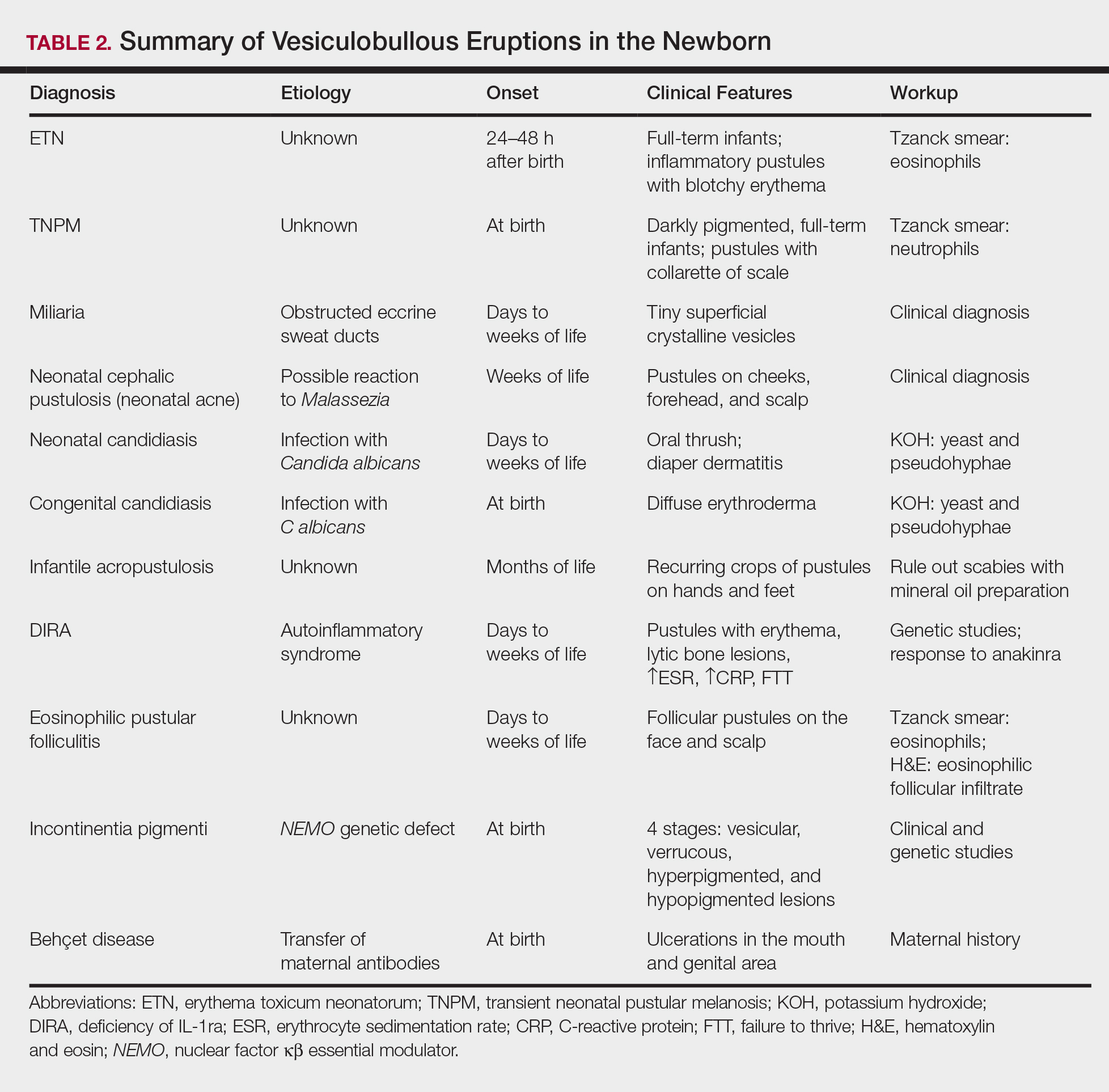
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Personal models of illness
Cognitive reappraisal is a top-down emotional regulation skill associated with resilience – the capacity to adaptively overcome adversity.
A person with this ability, also known as cognitive flexibility or reframing, monitors negative thoughts or situations and intentionally changes the way he or she views them. This reframing can involve retaining a positive outlook, trying to create meaning from a difficult situation, or finding ways to exert control over specific circumstances (Front Behav Neurosci. 2013 Feb 15;7:10). Some individuals cope with their mental illness by creating their own models of their illness (Achieving Cultural Competency: A Case-Based Approach to Training Health Professionals, Hoboken, N.J.: Wiley-Blackwell Publishing, 2009).
Creating a model of illness is a type of reframing to help explain what’s happening to an individual by placing the locus of control either inside our ourselves, adjacent, or far away and uncontrollable. Depending on the model, there might be choice that results in action taken to face the mental illness. Sometimes, there is surrender, either to the illness or the treatment.
In one of my weekly phone conversations with my mother in Texas, she told me that Ricardo, the husband of close family friend, had sunk into a deep depression to the point where he could no longer leave the house for work. Ricardo is an unauthorized immigrant, having crossed the border from Mexico into Texas 17 years ago with his wife and 2-year-old son. He lives a story common to many families in Texas: two undocumented parents working in local businesses, one child with a DACA (Deferred Action for Childhood Arrivals) permit and their second child born in the United States, all assimilated into American culture. With Ricardo’s descent into personal darkness, their American dream was fraying. Family and neighbors were gossiping about what could have happened – had Ricardo gotten into trouble with drugs and alcohol? Perhaps his wife had bewitched him; perhaps this was a godly test that only prayer could overcome.
I called his wife to see if I could offer her help navigating the local mental health system. She recounted a story of severe depression, and, most worryingly, a recent self-aborted hanging. Because of cultural beliefs, stigma of mental illness, and his immigration status, Ricardo would not call the local mental health authority for assessment and treatment.
So I made a trip to Texas to see Ricardo as a friend and psychiatrist, despite not quite knowing how to navigate the moral and legal ambiguity of this situation. I could at least offer a comprehensive psychiatric assessment and provide him with some understanding of his illness to help guide his decisions. My conversation with Ricardo found a man helpless and confused as to how and why he lost all drive, energy, and desire to live. We spoke about his and my understanding of depression. I tried to help Ricardo by shifting his perception of his illness from fear of an unknown specter to the idea that his current state of mind could be attributed to a treatable brain disease.
The trip to Texas was also an opportunity to see my older brother’s newly purchased home. This was a serious achievement, following 2 years where he had lived with our parents to save money for a down payment. He had initially been forced to live at home because of legal consequences related to his struggles with addiction and depression, both backdrops to his life as a devoted math teacher. In the car ride to his new house, he told me about his twice weekly, state-mandated addiction counseling group sessions. He has benefited from the instruction to fill his sober time with positive forces, telling me that he could not have bought his house and started working a second, part-time job without his sobriety.
Yet, he disagrees when the counselor tells his class that addiction is a disease that compromises his free will, and compared to his peers, he has less control over his mind when exposed to alcohol. He says it’s a mixed message – be proactive and take control over a new sober life, but be careful, your brain is too weak and diseased to ever have a healthy relationship with alcohol.
I was affected when he told me that he was afraid to ever drink again; that he cannot trust himself. He is afraid to fail and lose the life he is building for himself. Now he lives in conflict between two models of his illness: the determinism of addiction versus free will to overcome his abusive relationship with alcohol. To overcome this conflict, he has surrendered himself to a self-designed treatment program, working two jobs to fill his days and nights, and guarantee fatigue and sleep by the end of the day. No time to think or drink; just time to work and sleep.
The night before I flew to Texas, I had an overnight call in the emergency department. I encountered a young woman whom I’ll call Laura. She was in her mid 30s with HIV/AIDS with a CD4 count of less than 30, and had not taken medication for her HIV in years. Mostly, she lived in and out of hospitals, both psychiatric and medical wards. I was called to assess her suicidal ideation with a stated plan to slip and fall in her shower in order to hit her head and die. She was cachectic, tired, withdrawn, disheveled, buried under a heap of blankets.
Our interview was an awkward dance around why she could not and would not take medications for either her HIV/AIDS or posttraumatic stress disorder and depression. No money, no transport, intermittently homeless, no desire to live nor a future to live for.
In our conversation, I searched for reasons for Laura to live, and she countered with reasons why it was easier to die. It was a level of apathy I have encountered with other severely ill AIDS patients – the brain is so immunocompromised and muddled, the body so tired, the spirit so damaged. Her three children living with a sister had lost their potency as motivation to desire recovery of her physical and mental health. I doubted the active nature of her suicidality, and her apathy and physical deterioration made me question her ability to act on a plan. Nonetheless, I admitted Laura to the psychiatric unit for safety. Two weeks later, I learned she had died in hospital of AIDS-related sepsis. She had 10 days of treatment on the psychiatric unit with no movement in her depressive symptoms and apathy. Eventually, she physically crashed and was sent to the ICU, where she died.
As psychiatrists, we create our own models of what mental illness and treatments are, and we apply some version of the model to each patient. With the concepts of cultural psychiatry and therapeutic alliance, we learn to work within our patients’ models of disease to enhance their response to treatment. My initial reaction to Laura’s death was surprise, fear, and guilt that maybe I had missed a pressing medical issue that contributed to her death. Then I just felt resigned to her death, probably as she did. She told me in the emergency department she was set on dying, and her actions, well before this last admission, had indirectly ensured an early death. We psychiatrists feel failure when we are unable to prevent a suicide. What was Laura’s death: Was it a suicide by apathy that a psychiatrist could have prevented? Or just an expected complication of an untreated chronic illness? Many residents had done their job by admitting her again and again for either psychiatric or medical illness. Yet none of us could understand why she refused to treat her HIV/AIDS, and none of us was able to address the model she had created of her illness. Her model, that her HIV was a death sentence, was anathema to our training.
Because of that dissonance, it was difficult to understand her narrative, let alone find a way to help her reframe it. Her model of illness was misunderstood by a wide swathe of medical professionals, and together we were unable to tailor a treatment to her needs. Since, I’ve worked to reframe her death in my own mind as a way to better understand models of illness, learning from her as well as from my brother and my friend Ricardo. Both the patient’s and physician’s conceptualization of illness affects prognosis of whether to surrender to a treatment or the illness. As psychiatrists, we must strive to understand all models of illness, so we can plan and implement our treatment intervention accordingly.
I asked my friend from home and my brother for their permission and sent them this piece to make sure they approved. I changed certain details about Ricardo’s story to protect his identity. With my brother, there was no way to change his identity, but he was touched and happy to be included. I also changed key facts about the patient I called Laura.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
Cognitive reappraisal is a top-down emotional regulation skill associated with resilience – the capacity to adaptively overcome adversity.
A person with this ability, also known as cognitive flexibility or reframing, monitors negative thoughts or situations and intentionally changes the way he or she views them. This reframing can involve retaining a positive outlook, trying to create meaning from a difficult situation, or finding ways to exert control over specific circumstances (Front Behav Neurosci. 2013 Feb 15;7:10). Some individuals cope with their mental illness by creating their own models of their illness (Achieving Cultural Competency: A Case-Based Approach to Training Health Professionals, Hoboken, N.J.: Wiley-Blackwell Publishing, 2009).
Creating a model of illness is a type of reframing to help explain what’s happening to an individual by placing the locus of control either inside our ourselves, adjacent, or far away and uncontrollable. Depending on the model, there might be choice that results in action taken to face the mental illness. Sometimes, there is surrender, either to the illness or the treatment.
In one of my weekly phone conversations with my mother in Texas, she told me that Ricardo, the husband of close family friend, had sunk into a deep depression to the point where he could no longer leave the house for work. Ricardo is an unauthorized immigrant, having crossed the border from Mexico into Texas 17 years ago with his wife and 2-year-old son. He lives a story common to many families in Texas: two undocumented parents working in local businesses, one child with a DACA (Deferred Action for Childhood Arrivals) permit and their second child born in the United States, all assimilated into American culture. With Ricardo’s descent into personal darkness, their American dream was fraying. Family and neighbors were gossiping about what could have happened – had Ricardo gotten into trouble with drugs and alcohol? Perhaps his wife had bewitched him; perhaps this was a godly test that only prayer could overcome.
I called his wife to see if I could offer her help navigating the local mental health system. She recounted a story of severe depression, and, most worryingly, a recent self-aborted hanging. Because of cultural beliefs, stigma of mental illness, and his immigration status, Ricardo would not call the local mental health authority for assessment and treatment.
So I made a trip to Texas to see Ricardo as a friend and psychiatrist, despite not quite knowing how to navigate the moral and legal ambiguity of this situation. I could at least offer a comprehensive psychiatric assessment and provide him with some understanding of his illness to help guide his decisions. My conversation with Ricardo found a man helpless and confused as to how and why he lost all drive, energy, and desire to live. We spoke about his and my understanding of depression. I tried to help Ricardo by shifting his perception of his illness from fear of an unknown specter to the idea that his current state of mind could be attributed to a treatable brain disease.
The trip to Texas was also an opportunity to see my older brother’s newly purchased home. This was a serious achievement, following 2 years where he had lived with our parents to save money for a down payment. He had initially been forced to live at home because of legal consequences related to his struggles with addiction and depression, both backdrops to his life as a devoted math teacher. In the car ride to his new house, he told me about his twice weekly, state-mandated addiction counseling group sessions. He has benefited from the instruction to fill his sober time with positive forces, telling me that he could not have bought his house and started working a second, part-time job without his sobriety.
Yet, he disagrees when the counselor tells his class that addiction is a disease that compromises his free will, and compared to his peers, he has less control over his mind when exposed to alcohol. He says it’s a mixed message – be proactive and take control over a new sober life, but be careful, your brain is too weak and diseased to ever have a healthy relationship with alcohol.
I was affected when he told me that he was afraid to ever drink again; that he cannot trust himself. He is afraid to fail and lose the life he is building for himself. Now he lives in conflict between two models of his illness: the determinism of addiction versus free will to overcome his abusive relationship with alcohol. To overcome this conflict, he has surrendered himself to a self-designed treatment program, working two jobs to fill his days and nights, and guarantee fatigue and sleep by the end of the day. No time to think or drink; just time to work and sleep.
The night before I flew to Texas, I had an overnight call in the emergency department. I encountered a young woman whom I’ll call Laura. She was in her mid 30s with HIV/AIDS with a CD4 count of less than 30, and had not taken medication for her HIV in years. Mostly, she lived in and out of hospitals, both psychiatric and medical wards. I was called to assess her suicidal ideation with a stated plan to slip and fall in her shower in order to hit her head and die. She was cachectic, tired, withdrawn, disheveled, buried under a heap of blankets.
Our interview was an awkward dance around why she could not and would not take medications for either her HIV/AIDS or posttraumatic stress disorder and depression. No money, no transport, intermittently homeless, no desire to live nor a future to live for.
In our conversation, I searched for reasons for Laura to live, and she countered with reasons why it was easier to die. It was a level of apathy I have encountered with other severely ill AIDS patients – the brain is so immunocompromised and muddled, the body so tired, the spirit so damaged. Her three children living with a sister had lost their potency as motivation to desire recovery of her physical and mental health. I doubted the active nature of her suicidality, and her apathy and physical deterioration made me question her ability to act on a plan. Nonetheless, I admitted Laura to the psychiatric unit for safety. Two weeks later, I learned she had died in hospital of AIDS-related sepsis. She had 10 days of treatment on the psychiatric unit with no movement in her depressive symptoms and apathy. Eventually, she physically crashed and was sent to the ICU, where she died.
As psychiatrists, we create our own models of what mental illness and treatments are, and we apply some version of the model to each patient. With the concepts of cultural psychiatry and therapeutic alliance, we learn to work within our patients’ models of disease to enhance their response to treatment. My initial reaction to Laura’s death was surprise, fear, and guilt that maybe I had missed a pressing medical issue that contributed to her death. Then I just felt resigned to her death, probably as she did. She told me in the emergency department she was set on dying, and her actions, well before this last admission, had indirectly ensured an early death. We psychiatrists feel failure when we are unable to prevent a suicide. What was Laura’s death: Was it a suicide by apathy that a psychiatrist could have prevented? Or just an expected complication of an untreated chronic illness? Many residents had done their job by admitting her again and again for either psychiatric or medical illness. Yet none of us could understand why she refused to treat her HIV/AIDS, and none of us was able to address the model she had created of her illness. Her model, that her HIV was a death sentence, was anathema to our training.
Because of that dissonance, it was difficult to understand her narrative, let alone find a way to help her reframe it. Her model of illness was misunderstood by a wide swathe of medical professionals, and together we were unable to tailor a treatment to her needs. Since, I’ve worked to reframe her death in my own mind as a way to better understand models of illness, learning from her as well as from my brother and my friend Ricardo. Both the patient’s and physician’s conceptualization of illness affects prognosis of whether to surrender to a treatment or the illness. As psychiatrists, we must strive to understand all models of illness, so we can plan and implement our treatment intervention accordingly.
I asked my friend from home and my brother for their permission and sent them this piece to make sure they approved. I changed certain details about Ricardo’s story to protect his identity. With my brother, there was no way to change his identity, but he was touched and happy to be included. I also changed key facts about the patient I called Laura.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
Cognitive reappraisal is a top-down emotional regulation skill associated with resilience – the capacity to adaptively overcome adversity.
A person with this ability, also known as cognitive flexibility or reframing, monitors negative thoughts or situations and intentionally changes the way he or she views them. This reframing can involve retaining a positive outlook, trying to create meaning from a difficult situation, or finding ways to exert control over specific circumstances (Front Behav Neurosci. 2013 Feb 15;7:10). Some individuals cope with their mental illness by creating their own models of their illness (Achieving Cultural Competency: A Case-Based Approach to Training Health Professionals, Hoboken, N.J.: Wiley-Blackwell Publishing, 2009).
Creating a model of illness is a type of reframing to help explain what’s happening to an individual by placing the locus of control either inside our ourselves, adjacent, or far away and uncontrollable. Depending on the model, there might be choice that results in action taken to face the mental illness. Sometimes, there is surrender, either to the illness or the treatment.
In one of my weekly phone conversations with my mother in Texas, she told me that Ricardo, the husband of close family friend, had sunk into a deep depression to the point where he could no longer leave the house for work. Ricardo is an unauthorized immigrant, having crossed the border from Mexico into Texas 17 years ago with his wife and 2-year-old son. He lives a story common to many families in Texas: two undocumented parents working in local businesses, one child with a DACA (Deferred Action for Childhood Arrivals) permit and their second child born in the United States, all assimilated into American culture. With Ricardo’s descent into personal darkness, their American dream was fraying. Family and neighbors were gossiping about what could have happened – had Ricardo gotten into trouble with drugs and alcohol? Perhaps his wife had bewitched him; perhaps this was a godly test that only prayer could overcome.
I called his wife to see if I could offer her help navigating the local mental health system. She recounted a story of severe depression, and, most worryingly, a recent self-aborted hanging. Because of cultural beliefs, stigma of mental illness, and his immigration status, Ricardo would not call the local mental health authority for assessment and treatment.
So I made a trip to Texas to see Ricardo as a friend and psychiatrist, despite not quite knowing how to navigate the moral and legal ambiguity of this situation. I could at least offer a comprehensive psychiatric assessment and provide him with some understanding of his illness to help guide his decisions. My conversation with Ricardo found a man helpless and confused as to how and why he lost all drive, energy, and desire to live. We spoke about his and my understanding of depression. I tried to help Ricardo by shifting his perception of his illness from fear of an unknown specter to the idea that his current state of mind could be attributed to a treatable brain disease.
The trip to Texas was also an opportunity to see my older brother’s newly purchased home. This was a serious achievement, following 2 years where he had lived with our parents to save money for a down payment. He had initially been forced to live at home because of legal consequences related to his struggles with addiction and depression, both backdrops to his life as a devoted math teacher. In the car ride to his new house, he told me about his twice weekly, state-mandated addiction counseling group sessions. He has benefited from the instruction to fill his sober time with positive forces, telling me that he could not have bought his house and started working a second, part-time job without his sobriety.
Yet, he disagrees when the counselor tells his class that addiction is a disease that compromises his free will, and compared to his peers, he has less control over his mind when exposed to alcohol. He says it’s a mixed message – be proactive and take control over a new sober life, but be careful, your brain is too weak and diseased to ever have a healthy relationship with alcohol.
I was affected when he told me that he was afraid to ever drink again; that he cannot trust himself. He is afraid to fail and lose the life he is building for himself. Now he lives in conflict between two models of his illness: the determinism of addiction versus free will to overcome his abusive relationship with alcohol. To overcome this conflict, he has surrendered himself to a self-designed treatment program, working two jobs to fill his days and nights, and guarantee fatigue and sleep by the end of the day. No time to think or drink; just time to work and sleep.
The night before I flew to Texas, I had an overnight call in the emergency department. I encountered a young woman whom I’ll call Laura. She was in her mid 30s with HIV/AIDS with a CD4 count of less than 30, and had not taken medication for her HIV in years. Mostly, she lived in and out of hospitals, both psychiatric and medical wards. I was called to assess her suicidal ideation with a stated plan to slip and fall in her shower in order to hit her head and die. She was cachectic, tired, withdrawn, disheveled, buried under a heap of blankets.
Our interview was an awkward dance around why she could not and would not take medications for either her HIV/AIDS or posttraumatic stress disorder and depression. No money, no transport, intermittently homeless, no desire to live nor a future to live for.
In our conversation, I searched for reasons for Laura to live, and she countered with reasons why it was easier to die. It was a level of apathy I have encountered with other severely ill AIDS patients – the brain is so immunocompromised and muddled, the body so tired, the spirit so damaged. Her three children living with a sister had lost their potency as motivation to desire recovery of her physical and mental health. I doubted the active nature of her suicidality, and her apathy and physical deterioration made me question her ability to act on a plan. Nonetheless, I admitted Laura to the psychiatric unit for safety. Two weeks later, I learned she had died in hospital of AIDS-related sepsis. She had 10 days of treatment on the psychiatric unit with no movement in her depressive symptoms and apathy. Eventually, she physically crashed and was sent to the ICU, where she died.
As psychiatrists, we create our own models of what mental illness and treatments are, and we apply some version of the model to each patient. With the concepts of cultural psychiatry and therapeutic alliance, we learn to work within our patients’ models of disease to enhance their response to treatment. My initial reaction to Laura’s death was surprise, fear, and guilt that maybe I had missed a pressing medical issue that contributed to her death. Then I just felt resigned to her death, probably as she did. She told me in the emergency department she was set on dying, and her actions, well before this last admission, had indirectly ensured an early death. We psychiatrists feel failure when we are unable to prevent a suicide. What was Laura’s death: Was it a suicide by apathy that a psychiatrist could have prevented? Or just an expected complication of an untreated chronic illness? Many residents had done their job by admitting her again and again for either psychiatric or medical illness. Yet none of us could understand why she refused to treat her HIV/AIDS, and none of us was able to address the model she had created of her illness. Her model, that her HIV was a death sentence, was anathema to our training.
Because of that dissonance, it was difficult to understand her narrative, let alone find a way to help her reframe it. Her model of illness was misunderstood by a wide swathe of medical professionals, and together we were unable to tailor a treatment to her needs. Since, I’ve worked to reframe her death in my own mind as a way to better understand models of illness, learning from her as well as from my brother and my friend Ricardo. Both the patient’s and physician’s conceptualization of illness affects prognosis of whether to surrender to a treatment or the illness. As psychiatrists, we must strive to understand all models of illness, so we can plan and implement our treatment intervention accordingly.
I asked my friend from home and my brother for their permission and sent them this piece to make sure they approved. I changed certain details about Ricardo’s story to protect his identity. With my brother, there was no way to change his identity, but he was touched and happy to be included. I also changed key facts about the patient I called Laura.
Dr. Posada is a third-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, and health disparities, and she plans to pursue a fellowship in consult liaison psychiatry.
Pediatric Pearls From the AAD Annual Meeting
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
Do you talk about marijuana with your patients?
Do you talk about marijuana with your patients?
In medical school, I had a roommate. He was a smart law school graduate, good looking, outgoing, had lots of friends, was funny, and he was a great cook.
I should tell you another thing about my friend: He was a heavy, daily user of marijuana.
I believe that most of us, at a certain point of our lives, have met someone like my friend. The combination of a high-stress lifestyle, high self-expectations, and lack of appropriate skills to tackle life’s obstacles when encountered with failure frequently leads to addiction or a behavioral problem. In most cases, that will cause a pathological relationship with an outside substance or stimuli (Internet overuse/shopping too much/overeating or drinking, and so on).
Living a life filled with severe trauma and pain, especially at a developmental stage, often leads to an addiction. We frequently see people escape to the sweet narcotic-induced sleep via opioid abuse. On the other hand, for people who did not suffer trauma and are highly functional, marijuana offers a means of emotional detachment from pain, in its different form, and existential depression. That is the main benefit my patients who live with marijuana addiction get.
My friend serves as a rather stereotypical – and some may say – subjective, simplistic example of what is becoming more and more common in our society. I’m willing to bet that a good number of clinicians who read this have a similar example in mind.
With its intoxication state perceived as benign and the limited medicinal advantages, marijuana rapidly is gaining more and more legitimacy in the eyes of the general public (Addict Behav. 2008 Mar;33:397-411), (Monitoring the Future: National Results on Drug Use: 1975-2016). The risk of addiction is perceived as negligible and often nonexistent.
Almost no one knows about the potential risk of addiction (around 9%) (Drug Alcohol Depend. 2011;115:120-30). No one knows about about tolerance and withdrawal states – or about the real risk of psychosis (N Engl J Med. 2014 Jun 5;370:2219-27) or about the possible risk of schizophrenia in vulnerable populations (Schizophr Res. 2016 Mar;171:[1-3]:62-7). No one talks about the fact that it’s often used with tobacco. (How many times have you been told during history taking that a patient doesn’t smoke tobacco, only to find that in drug history he smokes 3-5 joints with tobacco daily?)
[polldaddy:9796432]
Throughout my journey in the psychiatric world (studying and working on three different continents) another typical marijuana user is the patient living with chronic mental illness. My Israeli mentor often complained about not having a single “clean” patient with schizophrenia anymore. I now see the same phenomena in Philadelphia and was also exposed to the same reality in Europe during medical school.
As physicians, and especially as psychiatrists, I believe we are obligated to educate our patients by telling them about the risks in their behaviors. Educating patients about marijuana in today’s atmosphere can be a very important preventive measure, and awareness is an important step toward change.
The current generation of psychiatrists is dealing with an opioid epidemic. Let’s educate ourselves and our patients so this current epidemic won’t be followed by another, severe cannabis epidemic.
Dr. Shilo is a second-year PGY in the department of psychiatry at Einstein Medical Center, Philadelphia.
Do you talk about marijuana with your patients?
In medical school, I had a roommate. He was a smart law school graduate, good looking, outgoing, had lots of friends, was funny, and he was a great cook.
I should tell you another thing about my friend: He was a heavy, daily user of marijuana.
I believe that most of us, at a certain point of our lives, have met someone like my friend. The combination of a high-stress lifestyle, high self-expectations, and lack of appropriate skills to tackle life’s obstacles when encountered with failure frequently leads to addiction or a behavioral problem. In most cases, that will cause a pathological relationship with an outside substance or stimuli (Internet overuse/shopping too much/overeating or drinking, and so on).
Living a life filled with severe trauma and pain, especially at a developmental stage, often leads to an addiction. We frequently see people escape to the sweet narcotic-induced sleep via opioid abuse. On the other hand, for people who did not suffer trauma and are highly functional, marijuana offers a means of emotional detachment from pain, in its different form, and existential depression. That is the main benefit my patients who live with marijuana addiction get.
My friend serves as a rather stereotypical – and some may say – subjective, simplistic example of what is becoming more and more common in our society. I’m willing to bet that a good number of clinicians who read this have a similar example in mind.
With its intoxication state perceived as benign and the limited medicinal advantages, marijuana rapidly is gaining more and more legitimacy in the eyes of the general public (Addict Behav. 2008 Mar;33:397-411), (Monitoring the Future: National Results on Drug Use: 1975-2016). The risk of addiction is perceived as negligible and often nonexistent.
Almost no one knows about the potential risk of addiction (around 9%) (Drug Alcohol Depend. 2011;115:120-30). No one knows about about tolerance and withdrawal states – or about the real risk of psychosis (N Engl J Med. 2014 Jun 5;370:2219-27) or about the possible risk of schizophrenia in vulnerable populations (Schizophr Res. 2016 Mar;171:[1-3]:62-7). No one talks about the fact that it’s often used with tobacco. (How many times have you been told during history taking that a patient doesn’t smoke tobacco, only to find that in drug history he smokes 3-5 joints with tobacco daily?)
[polldaddy:9796432]
Throughout my journey in the psychiatric world (studying and working on three different continents) another typical marijuana user is the patient living with chronic mental illness. My Israeli mentor often complained about not having a single “clean” patient with schizophrenia anymore. I now see the same phenomena in Philadelphia and was also exposed to the same reality in Europe during medical school.
As physicians, and especially as psychiatrists, I believe we are obligated to educate our patients by telling them about the risks in their behaviors. Educating patients about marijuana in today’s atmosphere can be a very important preventive measure, and awareness is an important step toward change.
The current generation of psychiatrists is dealing with an opioid epidemic. Let’s educate ourselves and our patients so this current epidemic won’t be followed by another, severe cannabis epidemic.
Dr. Shilo is a second-year PGY in the department of psychiatry at Einstein Medical Center, Philadelphia.
Do you talk about marijuana with your patients?
In medical school, I had a roommate. He was a smart law school graduate, good looking, outgoing, had lots of friends, was funny, and he was a great cook.
I should tell you another thing about my friend: He was a heavy, daily user of marijuana.
I believe that most of us, at a certain point of our lives, have met someone like my friend. The combination of a high-stress lifestyle, high self-expectations, and lack of appropriate skills to tackle life’s obstacles when encountered with failure frequently leads to addiction or a behavioral problem. In most cases, that will cause a pathological relationship with an outside substance or stimuli (Internet overuse/shopping too much/overeating or drinking, and so on).
Living a life filled with severe trauma and pain, especially at a developmental stage, often leads to an addiction. We frequently see people escape to the sweet narcotic-induced sleep via opioid abuse. On the other hand, for people who did not suffer trauma and are highly functional, marijuana offers a means of emotional detachment from pain, in its different form, and existential depression. That is the main benefit my patients who live with marijuana addiction get.
My friend serves as a rather stereotypical – and some may say – subjective, simplistic example of what is becoming more and more common in our society. I’m willing to bet that a good number of clinicians who read this have a similar example in mind.
With its intoxication state perceived as benign and the limited medicinal advantages, marijuana rapidly is gaining more and more legitimacy in the eyes of the general public (Addict Behav. 2008 Mar;33:397-411), (Monitoring the Future: National Results on Drug Use: 1975-2016). The risk of addiction is perceived as negligible and often nonexistent.
Almost no one knows about the potential risk of addiction (around 9%) (Drug Alcohol Depend. 2011;115:120-30). No one knows about about tolerance and withdrawal states – or about the real risk of psychosis (N Engl J Med. 2014 Jun 5;370:2219-27) or about the possible risk of schizophrenia in vulnerable populations (Schizophr Res. 2016 Mar;171:[1-3]:62-7). No one talks about the fact that it’s often used with tobacco. (How many times have you been told during history taking that a patient doesn’t smoke tobacco, only to find that in drug history he smokes 3-5 joints with tobacco daily?)
[polldaddy:9796432]
Throughout my journey in the psychiatric world (studying and working on three different continents) another typical marijuana user is the patient living with chronic mental illness. My Israeli mentor often complained about not having a single “clean” patient with schizophrenia anymore. I now see the same phenomena in Philadelphia and was also exposed to the same reality in Europe during medical school.
As physicians, and especially as psychiatrists, I believe we are obligated to educate our patients by telling them about the risks in their behaviors. Educating patients about marijuana in today’s atmosphere can be a very important preventive measure, and awareness is an important step toward change.
The current generation of psychiatrists is dealing with an opioid epidemic. Let’s educate ourselves and our patients so this current epidemic won’t be followed by another, severe cannabis epidemic.
Dr. Shilo is a second-year PGY in the department of psychiatry at Einstein Medical Center, Philadelphia.
Transgender patients face mental health challenges
What I thought would be a typical morning at the major county hospital – if that were ever possible on a psychiatric emergency unit (EU) – quickly turned atypical when I encountered a patient whom I will call Olivia.
Olivia is a 51-year-old male-to-female transgender woman who has not yet undergone gender reassignment surgery. She was brought to the EU by police, after expressing suicidal ideation during a phone call with a tax accountant. This was Olivia’s third visit to the psychiatric emergency unit because of suicide threats within 2 months. Though she was chronically suicidal, her current visit was triggered by a tax audit, which led her to view herself as an unwanted burden on society.
As a male-to-female transgender woman, Olivia faced discrimination in various aspects of her life. Despite her professional competence as an accountant, she was unable to find an employer accepting of her transgender status. Her efforts to align her legal identity with her experienced gender by legally changing her name were met by a web of bureaucratic complications. In the face of numerous challenges, Olivia had minimal social support to rely on during her gender-affirming transition. In short, nothing – not work, family, or finances – was simple for Olivia.
Olivia’s case is not atypical. My purpose in describing Olivia’s circumstances is to highlight the issues faced by many transgender men and women in every aspect of their day-to-day lives. To protect her identity, I have been careful to change several specifics related to her case.
Historical perspective
Societal views of transgendered people vary. On one end of the spectrum is acceptance; in the middle is perhaps tolerance or mild discomfort at perceived abnormality; and at the other extreme are virulent hatred, discrimination, and invalidation of these individuals’ gender-affirming efforts.
Awareness of these hostile attitudes creates a vicious cycle of marginalization and mental illness among many transgender individuals.
Controversies surrounding sexual minorities are rooted in societal perceptions of gender delineations, and prevailing societal norms surrounding ethical and moral conduct. Most societies have a narrow perspective on gender, and seek to maintain a strict delineation of male and female identities, which contributes to the rejection of those with gender identity issues.1,2 This often results in the invalidation of or active opposition to transgender individuals’ transition efforts. The reasons behind the rejection of transgender men and women are multifactorial, and can include a lack of awareness, homophobia, religious dogma, social stigmatization, and perceived non-employability – all often stemming largely from the lack of awareness that sexual identity and orientation are not a choice, but rather are predetermined by biological mechanisms. The intensifying familial, societal, political, and financial pressures all contribute to increased mental health issues, including increased incidence of suicide among sexual minorities.
Globally, sexual minorities continue to be subjected to gross human rights violations. Against the backdrop of prevalent social discrimination, transgender people experience a roughly threefold increase in the risk of developing anxiety disorders, which impair self-esteem and interpersonal functioning.3 The lifetime risk of attempted suicide is four times higher among transgender men, and two times higher among transgender women, than it is among cisgender men and women, respectively. Institutional discrimination in the public and private sectors results from laws and policies that impose inequities, or fail to protect sexual minorities. Examples are current policies denying medical coverage for sex reassignment surgery, denying health care by a provider because of transgender identity and numerous obstacles to obtain health insurance coverage.4
Impact of low acceptance
Recall Olivia’s need to delay the vocal cord surgery that arguably would have had a positive impact on her self-esteem. The transgender population faces increased health risks and barriers to appropriate mental health evaluation and inclusive care, particularly individuals with low acceptance from family, friends, and partners.5
A century ago, changing one’s gender was considered both highly disreputable and an impossible feat.6 Today, sex reassignment is the focus of political debate, with activists seeking equal rights for transgender individuals, despite the high rate of mental disorders in the community. While there is some positive public perception of transgender people, most still hold religious or moral objections to sex reassignment. Olivia’s family, for example, is typical in their rejection of her gender-affirming efforts. One example of this rejection is forbidding her to wear women’s clothing, stating that it makes them feel ashamed and subjects them to social ridicule.
As a result, Olivia lacks the social support that could work to remedy, to some extent, her suicidal ideation. Efforts to alleviate financial burdens that result from workplace discrimination, impediments to the pursuit of health, security, and happiness and the bureaucratic hurdles to gender affirmation are needed.7,8 According to research by Kristen Clements-Nolle, PhD, MPH, and her associates, suicidality may be largely a reaction to the absence of legally secure equal rights for transgender men and women.9 In Olivia’s case, financial struggles with her mortgage, medical expenses for her autistic son, and anxiety about potentially losing the disability benefits on which she depends have added to her insecurity.
Financial insecurity resulting from her unemployment likely has exacerbated her feelings of inadequacy and depression. For example, because of her lack of financial resources, she had to delay the vocal cord surgery she desired. What would her prognosis be if her legal rights, including employment protection, were firmly in place? Likewise, the demands of parenting an autistic son posed another significant stressor that likely contributes to reciprocal stress for the child, resulting in the worsening of his autism symptoms.
In summary, transgender individuals face bias and discrimination in response to their gender-affirming efforts, which creates a vicious cycle of mental illness, suicidality, and societal marginalization. Addressing these endemic issues requires a multifaceted approach. Preventive strategies, including identification of mental health issues, and integration of mental health service with primary health care, are needed. Case registration, as a research measure to help understand the prevalence and severity of suicide among the LGBT population, would be beneficial.
Monitoring and follow-up of identified cases for supportive care (for example, gatekeeper training similar to the World Health Organization’s Suicide Prevention Initiative) also are needed to identify protective factors, in order to foster resilience in LGBT individuals facing negative reactions to disclosure of their sexual minority status. Legislation aimed at better facilitating a seamless integration of transgender men and women into mainstream society also is necessary. Supportive measures, particularly social supports promoting better mental health in trans individuals, could help reduce suicide rates. Finally, governmental initiatives to protect the human and constitutional rights of transgender people are key to minimizing the incidence of mental health issues and suicides among this vulnerable sexual minority group.
The path to addressing the issues faced by transgender individuals begins with education, which then leads to understanding. From understanding comes acceptance. Acceptance leads to equality – social, legal, and thereby, economic. Ensuring acceptance of all sexes as equal could mitigate the marginalization – in all its forms – experienced by gender-affirming individuals, with the end result being less mental illness and reduced rates of suicidality in this vulnerable population.7
Dr. Ahmed is a second-year resident in the department of psychiatry at the Nassau University Medical Center, East Meadow, New York. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and addiction psychiatry. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
References
1. LGBT Health. 2014 Jun;1(2):98-106.
2. Signs: Journal of Women in Culture and Society. 2006;32(1):83-111.
3. Int J Transgenderism. 2016:18(1):16-26.
4. Am J Public Health. 2014 Mar;104(3):e31-8.
5. Int J Transgenderism. 2017;18(1):104-18.
6. “Suicide attempts among transgender and gender non-conforming adults,” American Foundation for Suicide Prevention, 2014.
7. “Injustice at every turn: A report of the national transgender discrimination survey,” National Center for Transgender Equality, 2011.
8. J Gay Lesbian Soc Serv. 2014;26(2):186-206.
9. J Homosex. 2006;51(3):53-69.
What I thought would be a typical morning at the major county hospital – if that were ever possible on a psychiatric emergency unit (EU) – quickly turned atypical when I encountered a patient whom I will call Olivia.
Olivia is a 51-year-old male-to-female transgender woman who has not yet undergone gender reassignment surgery. She was brought to the EU by police, after expressing suicidal ideation during a phone call with a tax accountant. This was Olivia’s third visit to the psychiatric emergency unit because of suicide threats within 2 months. Though she was chronically suicidal, her current visit was triggered by a tax audit, which led her to view herself as an unwanted burden on society.
As a male-to-female transgender woman, Olivia faced discrimination in various aspects of her life. Despite her professional competence as an accountant, she was unable to find an employer accepting of her transgender status. Her efforts to align her legal identity with her experienced gender by legally changing her name were met by a web of bureaucratic complications. In the face of numerous challenges, Olivia had minimal social support to rely on during her gender-affirming transition. In short, nothing – not work, family, or finances – was simple for Olivia.
Olivia’s case is not atypical. My purpose in describing Olivia’s circumstances is to highlight the issues faced by many transgender men and women in every aspect of their day-to-day lives. To protect her identity, I have been careful to change several specifics related to her case.
Historical perspective
Societal views of transgendered people vary. On one end of the spectrum is acceptance; in the middle is perhaps tolerance or mild discomfort at perceived abnormality; and at the other extreme are virulent hatred, discrimination, and invalidation of these individuals’ gender-affirming efforts.
Awareness of these hostile attitudes creates a vicious cycle of marginalization and mental illness among many transgender individuals.
Controversies surrounding sexual minorities are rooted in societal perceptions of gender delineations, and prevailing societal norms surrounding ethical and moral conduct. Most societies have a narrow perspective on gender, and seek to maintain a strict delineation of male and female identities, which contributes to the rejection of those with gender identity issues.1,2 This often results in the invalidation of or active opposition to transgender individuals’ transition efforts. The reasons behind the rejection of transgender men and women are multifactorial, and can include a lack of awareness, homophobia, religious dogma, social stigmatization, and perceived non-employability – all often stemming largely from the lack of awareness that sexual identity and orientation are not a choice, but rather are predetermined by biological mechanisms. The intensifying familial, societal, political, and financial pressures all contribute to increased mental health issues, including increased incidence of suicide among sexual minorities.
Globally, sexual minorities continue to be subjected to gross human rights violations. Against the backdrop of prevalent social discrimination, transgender people experience a roughly threefold increase in the risk of developing anxiety disorders, which impair self-esteem and interpersonal functioning.3 The lifetime risk of attempted suicide is four times higher among transgender men, and two times higher among transgender women, than it is among cisgender men and women, respectively. Institutional discrimination in the public and private sectors results from laws and policies that impose inequities, or fail to protect sexual minorities. Examples are current policies denying medical coverage for sex reassignment surgery, denying health care by a provider because of transgender identity and numerous obstacles to obtain health insurance coverage.4
Impact of low acceptance
Recall Olivia’s need to delay the vocal cord surgery that arguably would have had a positive impact on her self-esteem. The transgender population faces increased health risks and barriers to appropriate mental health evaluation and inclusive care, particularly individuals with low acceptance from family, friends, and partners.5
A century ago, changing one’s gender was considered both highly disreputable and an impossible feat.6 Today, sex reassignment is the focus of political debate, with activists seeking equal rights for transgender individuals, despite the high rate of mental disorders in the community. While there is some positive public perception of transgender people, most still hold religious or moral objections to sex reassignment. Olivia’s family, for example, is typical in their rejection of her gender-affirming efforts. One example of this rejection is forbidding her to wear women’s clothing, stating that it makes them feel ashamed and subjects them to social ridicule.
As a result, Olivia lacks the social support that could work to remedy, to some extent, her suicidal ideation. Efforts to alleviate financial burdens that result from workplace discrimination, impediments to the pursuit of health, security, and happiness and the bureaucratic hurdles to gender affirmation are needed.7,8 According to research by Kristen Clements-Nolle, PhD, MPH, and her associates, suicidality may be largely a reaction to the absence of legally secure equal rights for transgender men and women.9 In Olivia’s case, financial struggles with her mortgage, medical expenses for her autistic son, and anxiety about potentially losing the disability benefits on which she depends have added to her insecurity.
Financial insecurity resulting from her unemployment likely has exacerbated her feelings of inadequacy and depression. For example, because of her lack of financial resources, she had to delay the vocal cord surgery she desired. What would her prognosis be if her legal rights, including employment protection, were firmly in place? Likewise, the demands of parenting an autistic son posed another significant stressor that likely contributes to reciprocal stress for the child, resulting in the worsening of his autism symptoms.
In summary, transgender individuals face bias and discrimination in response to their gender-affirming efforts, which creates a vicious cycle of mental illness, suicidality, and societal marginalization. Addressing these endemic issues requires a multifaceted approach. Preventive strategies, including identification of mental health issues, and integration of mental health service with primary health care, are needed. Case registration, as a research measure to help understand the prevalence and severity of suicide among the LGBT population, would be beneficial.
Monitoring and follow-up of identified cases for supportive care (for example, gatekeeper training similar to the World Health Organization’s Suicide Prevention Initiative) also are needed to identify protective factors, in order to foster resilience in LGBT individuals facing negative reactions to disclosure of their sexual minority status. Legislation aimed at better facilitating a seamless integration of transgender men and women into mainstream society also is necessary. Supportive measures, particularly social supports promoting better mental health in trans individuals, could help reduce suicide rates. Finally, governmental initiatives to protect the human and constitutional rights of transgender people are key to minimizing the incidence of mental health issues and suicides among this vulnerable sexual minority group.
The path to addressing the issues faced by transgender individuals begins with education, which then leads to understanding. From understanding comes acceptance. Acceptance leads to equality – social, legal, and thereby, economic. Ensuring acceptance of all sexes as equal could mitigate the marginalization – in all its forms – experienced by gender-affirming individuals, with the end result being less mental illness and reduced rates of suicidality in this vulnerable population.7
Dr. Ahmed is a second-year resident in the department of psychiatry at the Nassau University Medical Center, East Meadow, New York. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and addiction psychiatry. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
References
1. LGBT Health. 2014 Jun;1(2):98-106.
2. Signs: Journal of Women in Culture and Society. 2006;32(1):83-111.
3. Int J Transgenderism. 2016:18(1):16-26.
4. Am J Public Health. 2014 Mar;104(3):e31-8.
5. Int J Transgenderism. 2017;18(1):104-18.
6. “Suicide attempts among transgender and gender non-conforming adults,” American Foundation for Suicide Prevention, 2014.
7. “Injustice at every turn: A report of the national transgender discrimination survey,” National Center for Transgender Equality, 2011.
8. J Gay Lesbian Soc Serv. 2014;26(2):186-206.
9. J Homosex. 2006;51(3):53-69.
What I thought would be a typical morning at the major county hospital – if that were ever possible on a psychiatric emergency unit (EU) – quickly turned atypical when I encountered a patient whom I will call Olivia.
Olivia is a 51-year-old male-to-female transgender woman who has not yet undergone gender reassignment surgery. She was brought to the EU by police, after expressing suicidal ideation during a phone call with a tax accountant. This was Olivia’s third visit to the psychiatric emergency unit because of suicide threats within 2 months. Though she was chronically suicidal, her current visit was triggered by a tax audit, which led her to view herself as an unwanted burden on society.
As a male-to-female transgender woman, Olivia faced discrimination in various aspects of her life. Despite her professional competence as an accountant, she was unable to find an employer accepting of her transgender status. Her efforts to align her legal identity with her experienced gender by legally changing her name were met by a web of bureaucratic complications. In the face of numerous challenges, Olivia had minimal social support to rely on during her gender-affirming transition. In short, nothing – not work, family, or finances – was simple for Olivia.
Olivia’s case is not atypical. My purpose in describing Olivia’s circumstances is to highlight the issues faced by many transgender men and women in every aspect of their day-to-day lives. To protect her identity, I have been careful to change several specifics related to her case.
Historical perspective
Societal views of transgendered people vary. On one end of the spectrum is acceptance; in the middle is perhaps tolerance or mild discomfort at perceived abnormality; and at the other extreme are virulent hatred, discrimination, and invalidation of these individuals’ gender-affirming efforts.
Awareness of these hostile attitudes creates a vicious cycle of marginalization and mental illness among many transgender individuals.
Controversies surrounding sexual minorities are rooted in societal perceptions of gender delineations, and prevailing societal norms surrounding ethical and moral conduct. Most societies have a narrow perspective on gender, and seek to maintain a strict delineation of male and female identities, which contributes to the rejection of those with gender identity issues.1,2 This often results in the invalidation of or active opposition to transgender individuals’ transition efforts. The reasons behind the rejection of transgender men and women are multifactorial, and can include a lack of awareness, homophobia, religious dogma, social stigmatization, and perceived non-employability – all often stemming largely from the lack of awareness that sexual identity and orientation are not a choice, but rather are predetermined by biological mechanisms. The intensifying familial, societal, political, and financial pressures all contribute to increased mental health issues, including increased incidence of suicide among sexual minorities.
Globally, sexual minorities continue to be subjected to gross human rights violations. Against the backdrop of prevalent social discrimination, transgender people experience a roughly threefold increase in the risk of developing anxiety disorders, which impair self-esteem and interpersonal functioning.3 The lifetime risk of attempted suicide is four times higher among transgender men, and two times higher among transgender women, than it is among cisgender men and women, respectively. Institutional discrimination in the public and private sectors results from laws and policies that impose inequities, or fail to protect sexual minorities. Examples are current policies denying medical coverage for sex reassignment surgery, denying health care by a provider because of transgender identity and numerous obstacles to obtain health insurance coverage.4
Impact of low acceptance
Recall Olivia’s need to delay the vocal cord surgery that arguably would have had a positive impact on her self-esteem. The transgender population faces increased health risks and barriers to appropriate mental health evaluation and inclusive care, particularly individuals with low acceptance from family, friends, and partners.5
A century ago, changing one’s gender was considered both highly disreputable and an impossible feat.6 Today, sex reassignment is the focus of political debate, with activists seeking equal rights for transgender individuals, despite the high rate of mental disorders in the community. While there is some positive public perception of transgender people, most still hold religious or moral objections to sex reassignment. Olivia’s family, for example, is typical in their rejection of her gender-affirming efforts. One example of this rejection is forbidding her to wear women’s clothing, stating that it makes them feel ashamed and subjects them to social ridicule.
As a result, Olivia lacks the social support that could work to remedy, to some extent, her suicidal ideation. Efforts to alleviate financial burdens that result from workplace discrimination, impediments to the pursuit of health, security, and happiness and the bureaucratic hurdles to gender affirmation are needed.7,8 According to research by Kristen Clements-Nolle, PhD, MPH, and her associates, suicidality may be largely a reaction to the absence of legally secure equal rights for transgender men and women.9 In Olivia’s case, financial struggles with her mortgage, medical expenses for her autistic son, and anxiety about potentially losing the disability benefits on which she depends have added to her insecurity.
Financial insecurity resulting from her unemployment likely has exacerbated her feelings of inadequacy and depression. For example, because of her lack of financial resources, she had to delay the vocal cord surgery she desired. What would her prognosis be if her legal rights, including employment protection, were firmly in place? Likewise, the demands of parenting an autistic son posed another significant stressor that likely contributes to reciprocal stress for the child, resulting in the worsening of his autism symptoms.
In summary, transgender individuals face bias and discrimination in response to their gender-affirming efforts, which creates a vicious cycle of mental illness, suicidality, and societal marginalization. Addressing these endemic issues requires a multifaceted approach. Preventive strategies, including identification of mental health issues, and integration of mental health service with primary health care, are needed. Case registration, as a research measure to help understand the prevalence and severity of suicide among the LGBT population, would be beneficial.
Monitoring and follow-up of identified cases for supportive care (for example, gatekeeper training similar to the World Health Organization’s Suicide Prevention Initiative) also are needed to identify protective factors, in order to foster resilience in LGBT individuals facing negative reactions to disclosure of their sexual minority status. Legislation aimed at better facilitating a seamless integration of transgender men and women into mainstream society also is necessary. Supportive measures, particularly social supports promoting better mental health in trans individuals, could help reduce suicide rates. Finally, governmental initiatives to protect the human and constitutional rights of transgender people are key to minimizing the incidence of mental health issues and suicides among this vulnerable sexual minority group.
The path to addressing the issues faced by transgender individuals begins with education, which then leads to understanding. From understanding comes acceptance. Acceptance leads to equality – social, legal, and thereby, economic. Ensuring acceptance of all sexes as equal could mitigate the marginalization – in all its forms – experienced by gender-affirming individuals, with the end result being less mental illness and reduced rates of suicidality in this vulnerable population.7
Dr. Ahmed is a second-year resident in the department of psychiatry at the Nassau University Medical Center, East Meadow, New York. His interests include public social psychiatry, health care policy, health disparities, mental health stigma, and addiction psychiatry. Dr. Ahmed is a member of the American Psychiatric Association, the American Society of Clinical Psychopharmacology, and the American Association for Social Psychiatry.
References
1. LGBT Health. 2014 Jun;1(2):98-106.
2. Signs: Journal of Women in Culture and Society. 2006;32(1):83-111.
3. Int J Transgenderism. 2016:18(1):16-26.
4. Am J Public Health. 2014 Mar;104(3):e31-8.
5. Int J Transgenderism. 2017;18(1):104-18.
6. “Suicide attempts among transgender and gender non-conforming adults,” American Foundation for Suicide Prevention, 2014.
7. “Injustice at every turn: A report of the national transgender discrimination survey,” National Center for Transgender Equality, 2011.
8. J Gay Lesbian Soc Serv. 2014;26(2):186-206.
9. J Homosex. 2006;51(3):53-69.
Five Steps for Delivering an Effective and Educational Lecture
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
Expanding Uses of Propranolol in Dermatology
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
The challenges of dating
Dating during residency is a job unto itself – one that I didn’t expect to balance alongside my long and frequently odd hours of work. To make matters worse, I happen to be in a field where the examination is almost entirely through conversation, involving probing questions, where maintaining calm and strength is important in the face of patient stories that often tear at my heart. Then, on a date (first or otherwise) I spend more time listening to another person’s stories while trying hard not to interpret the information through the perspective of my profession. To orient my older readers, I primarily date using the Internet and dating applications. There, my profile names me as “Resident.” This title is unassuming and unintimidating. Resident of what, potential suitors wonder, while examining carefully curated pictures of travel to exotic places and of me smiling happily with friends. I swipe right and left, matching with people, chatting digitally at first, and, if there is mutual interest, arranging to meet in person.
I get a different reaction every time I tell a date I’m a psychiatrist. It seems each man has his own expectations of what it means to date a psychiatrist. This column is a shout-out to all the physicians, and especially psychiatrists, who have been single while practicing, required to pretend our work is just like everyone else’s. Because the truth is, our work is different. How can one really describe the goings-on at a psychiatric hospital to a potential partner? Or deal with fatigue that accompanies listening to emotional suffering while at work and then needing the energy to commiserate with the person across the table?
The second experience prompted this column – a recent breakup with a man I really felt and thought I could be with. I had begun perusing fellowships in the cities where he wanted to move. Perhaps predictably, my heart was broken. I am grateful to have a specialty that encourages a close connection to my patients. I am privileged to see and consider the motives, desires, fantasies, and fears of my patients. For dating, however, this skill is excessively fine tuned. I sense too subtly when something is going wrong. As a therapist in training, I am learning to hear the manifest content and listen for the latent content. I felt it with this man, despite knowing that he liked me and was serious about our relationship. I sensed the change in his approach to me – the transference, if you will. I suspected the reason was his stated desire to move to the West Coast “eventually.” When he actually revealed his plan to move and end the relationship, however, I cried as someone who has been hurt would cry. His response was: “ It will be okay. ... You’re supereligible. … Don’t cry. ... I’m surprised by all your emotion – especially the way you describe yourself at work.” He expected me to have control over my emotions because of my profession.
Psychiatrists are trained to examine emotions and behaviors, characterize them first as symptoms, then as diagnoses, and finally assess the best way to intervene. The emotions of my patients sometime get tangled with my own. I try to disentangle myself through my own therapy, and filling my life with productive activities and close friends. However, I think as a young, single psychiatrist, I have a space of loneliness that is too easily filled by the pain shared by my patients. I also expect better control over my emotions, but sometimes, my cup is already full, and all it can do is overflow with tears.
Dr. Posada is a second-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Dating during residency is a job unto itself – one that I didn’t expect to balance alongside my long and frequently odd hours of work. To make matters worse, I happen to be in a field where the examination is almost entirely through conversation, involving probing questions, where maintaining calm and strength is important in the face of patient stories that often tear at my heart. Then, on a date (first or otherwise) I spend more time listening to another person’s stories while trying hard not to interpret the information through the perspective of my profession. To orient my older readers, I primarily date using the Internet and dating applications. There, my profile names me as “Resident.” This title is unassuming and unintimidating. Resident of what, potential suitors wonder, while examining carefully curated pictures of travel to exotic places and of me smiling happily with friends. I swipe right and left, matching with people, chatting digitally at first, and, if there is mutual interest, arranging to meet in person.
I get a different reaction every time I tell a date I’m a psychiatrist. It seems each man has his own expectations of what it means to date a psychiatrist. This column is a shout-out to all the physicians, and especially psychiatrists, who have been single while practicing, required to pretend our work is just like everyone else’s. Because the truth is, our work is different. How can one really describe the goings-on at a psychiatric hospital to a potential partner? Or deal with fatigue that accompanies listening to emotional suffering while at work and then needing the energy to commiserate with the person across the table?
The second experience prompted this column – a recent breakup with a man I really felt and thought I could be with. I had begun perusing fellowships in the cities where he wanted to move. Perhaps predictably, my heart was broken. I am grateful to have a specialty that encourages a close connection to my patients. I am privileged to see and consider the motives, desires, fantasies, and fears of my patients. For dating, however, this skill is excessively fine tuned. I sense too subtly when something is going wrong. As a therapist in training, I am learning to hear the manifest content and listen for the latent content. I felt it with this man, despite knowing that he liked me and was serious about our relationship. I sensed the change in his approach to me – the transference, if you will. I suspected the reason was his stated desire to move to the West Coast “eventually.” When he actually revealed his plan to move and end the relationship, however, I cried as someone who has been hurt would cry. His response was: “ It will be okay. ... You’re supereligible. … Don’t cry. ... I’m surprised by all your emotion – especially the way you describe yourself at work.” He expected me to have control over my emotions because of my profession.
Psychiatrists are trained to examine emotions and behaviors, characterize them first as symptoms, then as diagnoses, and finally assess the best way to intervene. The emotions of my patients sometime get tangled with my own. I try to disentangle myself through my own therapy, and filling my life with productive activities and close friends. However, I think as a young, single psychiatrist, I have a space of loneliness that is too easily filled by the pain shared by my patients. I also expect better control over my emotions, but sometimes, my cup is already full, and all it can do is overflow with tears.
Dr. Posada is a second-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Dating during residency is a job unto itself – one that I didn’t expect to balance alongside my long and frequently odd hours of work. To make matters worse, I happen to be in a field where the examination is almost entirely through conversation, involving probing questions, where maintaining calm and strength is important in the face of patient stories that often tear at my heart. Then, on a date (first or otherwise) I spend more time listening to another person’s stories while trying hard not to interpret the information through the perspective of my profession. To orient my older readers, I primarily date using the Internet and dating applications. There, my profile names me as “Resident.” This title is unassuming and unintimidating. Resident of what, potential suitors wonder, while examining carefully curated pictures of travel to exotic places and of me smiling happily with friends. I swipe right and left, matching with people, chatting digitally at first, and, if there is mutual interest, arranging to meet in person.
I get a different reaction every time I tell a date I’m a psychiatrist. It seems each man has his own expectations of what it means to date a psychiatrist. This column is a shout-out to all the physicians, and especially psychiatrists, who have been single while practicing, required to pretend our work is just like everyone else’s. Because the truth is, our work is different. How can one really describe the goings-on at a psychiatric hospital to a potential partner? Or deal with fatigue that accompanies listening to emotional suffering while at work and then needing the energy to commiserate with the person across the table?
The second experience prompted this column – a recent breakup with a man I really felt and thought I could be with. I had begun perusing fellowships in the cities where he wanted to move. Perhaps predictably, my heart was broken. I am grateful to have a specialty that encourages a close connection to my patients. I am privileged to see and consider the motives, desires, fantasies, and fears of my patients. For dating, however, this skill is excessively fine tuned. I sense too subtly when something is going wrong. As a therapist in training, I am learning to hear the manifest content and listen for the latent content. I felt it with this man, despite knowing that he liked me and was serious about our relationship. I sensed the change in his approach to me – the transference, if you will. I suspected the reason was his stated desire to move to the West Coast “eventually.” When he actually revealed his plan to move and end the relationship, however, I cried as someone who has been hurt would cry. His response was: “ It will be okay. ... You’re supereligible. … Don’t cry. ... I’m surprised by all your emotion – especially the way you describe yourself at work.” He expected me to have control over my emotions because of my profession.
Psychiatrists are trained to examine emotions and behaviors, characterize them first as symptoms, then as diagnoses, and finally assess the best way to intervene. The emotions of my patients sometime get tangled with my own. I try to disentangle myself through my own therapy, and filling my life with productive activities and close friends. However, I think as a young, single psychiatrist, I have a space of loneliness that is too easily filled by the pain shared by my patients. I also expect better control over my emotions, but sometimes, my cup is already full, and all it can do is overflow with tears.
Dr. Posada is a second-year resident in the psychiatry and behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Pediatric Nail Diseases: Clinical Pearls
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.
Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3
Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.

Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3

Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.

Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3

Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
Developing a personal scale for evaluating agitation on inpatient units
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.